Potassium carbonate melting point
Home » datasheet » Potassium carbonate melting pointPotassium carbonate melting point
Potassium Carbonate Melting Point. K 2 CO 3 Uses Potassium carbonate Potassium carbonate is used as a mild drying agent. The chemical symbol K comes from kalium the Mediaeval Latin for potash which may have derived from the. Potassium Carbonate Structure K2CO3. It is used mainly in the form of a saturated solution 100 g potassium iodide to 100 ml water.
 Potassium Carbonate 584 08 7 From chemicalbook.com
Potassium Carbonate 584 08 7 From chemicalbook.com
The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. Potassium carbonate is mainly used in the production of soap and glass. That equates to around 50 mg drop. 497-19-8 Sodium carbonate. To do this he used the. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK.
CID 5462222 Potassium CID 767 Carbonic acid Dates.
Potassium Carbonate Structure K 2 CO 3. The idea of applying finely-grained rock to farmland is not new and several studies have shown that by-products from mines or quarries can improve soil quality. Melting Point of Potassium carbonate. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Potassium iodide KI is prepared by reacting potassium hydroxide to iodine with a hot solution. It is a white salt which is soluble in water.
 Source: sciencemadness.org
Source: sciencemadness.org
Potassium Carbonate Structure K2CO3. Melting Point of Potassium carbonate. It is produced by. The alkali metals are so called because reaction with water forms alkalies ie strong bases capable of neutralizing acidsSodium and potassium are the sixth and seventh most abundant of the elements. Substance Formula Melting point C Boiling temperature C Density 25C.
 Source: researchgate.net
Source: researchgate.net
We say that such a body melts. It is deliquescent often appearing as a damp or wet solid. These chemicals meet the FDA requirements for food use. The idea of applying finely-grained rock to farmland is not new and several studies have shown that by-products from mines or quarries can improve soil quality. Until drinking the solution is normally applied to the tea fruit juice or milk.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Potassium has two oxidation states 1 and -1rarely. CID 5462222 Potassium CID 767 Carbonic acid Dates. Sir Davy in 1808. Stable at room. But the method has gained interest due to the added benefit of absorbing CO2.
 Source: chemicalbook.com
Source: chemicalbook.com
Potassium bicarbonate is a white crystalline slightly alkaline and salty substance. Energy of first ionisation. CID 767 Carbonic acid Component Compounds. Until drinking the solution is normally applied to the tea fruit juice or milk. That realisation has been a.
 Source: byjus.com
Source: byjus.com
891 C 1636 F. Section 10 - Stability and Reactivity Chemical Stability. The idea of applying finely-grained rock to farmland is not new and several studies have shown that by-products from mines or quarries can improve soil quality. Potassium has two oxidation states 1 and -1rarely. Potassium Carbonate Structure K2CO3.

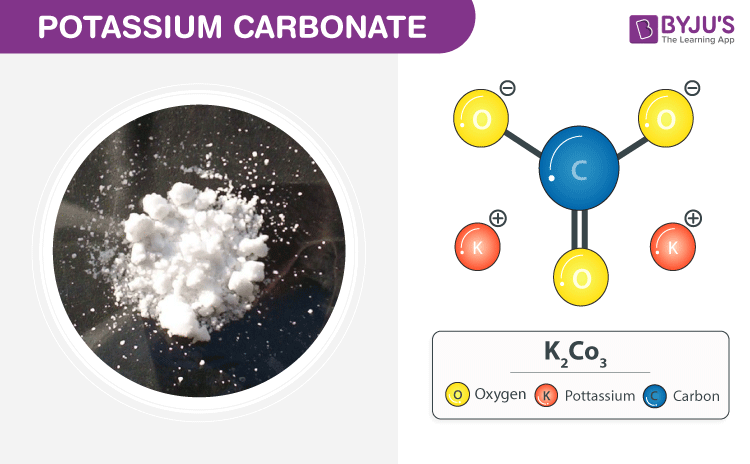
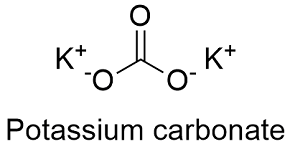
It constitutes 26 percent of Earths mass. Food Chemical Codex FCC. Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solutionIt can be made as the product of potassium hydroxides absorbent reaction with carbon dioxideIt presents a large capacity to absorb moisture. Potassium carbonate is mainly used in the production of soap and glass. Potassium bicarbonate is a white crystalline slightly alkaline and salty substance.
 Source: softschools.com
Source: softschools.com
Potassium bicarbonate is a white crystalline slightly alkaline and salty substance. K 2 CO 3 Uses Potassium carbonate Potassium carbonate is used as a mild drying agent. Potassium carbonate is the inorganic compound with the formula K 2 CO 3. It is a source of nitrogen and nitrogen was named after niterPotassium nitrate is one of several nitrogen-containing compounds. Food Chemical Codex FCC.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Potassium iodide KI is prepared by reacting potassium hydroxide to iodine with a hot solution. Used to produce Dutch process chocolate by alkalization. It is used mainly in the form of a saturated solution 100 g potassium iodide to 100 ml water. The idea of applying finely-grained rock to farmland is not new and several studies have shown that by-products from mines or quarries can improve soil quality. Potassium has two oxidation states 1 and -1rarely.
 Source: sciencedirect.com
Source: sciencedirect.com
The chemical symbol K comes from kalium the Mediaeval Latin for potash which may have derived from the. Melting point - the temperature at which a solid turns into a liquid. To do this he used the. The idea of applying finely-grained rock to farmland is not new and several studies have shown that by-products from mines or quarries can improve soil quality. Used in the production of wire or mead by acting as a buffering agent.
 Source: en.wikipedia.org
Source: en.wikipedia.org
759C 1398F 1032 K. Food Chemical Codex FCC. It is a white salt which is soluble in water. Potassium iodide KI is prepared by reacting potassium hydroxide to iodine with a hot solution. CID 5462222 Potassium CID 767 Carbonic acid Dates.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium carbonate melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.