Potassium carbonate boiling point
Home » datasheet » Potassium carbonate boiling pointPotassium carbonate boiling point
Potassium Carbonate Boiling Point. Potassium carbonate is mainly used in the production of soap and glass. The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. The solubility of potassium dichromate in the water at different temperatures is 49 g100 mL at 0 C and 13 g100 mL 20 C and 102 g100 mL at 100 C which means it becomes highly soluble in water at higher temperatures. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
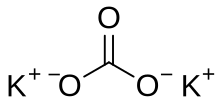 Potassium Carbonate Wikipedia From en.wikipedia.org
Potassium Carbonate Wikipedia From en.wikipedia.org
It is deliquescent often appearing as a damp or wet solid. Food Chemical Codex FCC. Use a limited amount. Energy of first ionisation. The vinegar causes the carbonate to be separated from the calcium. Boiling Point of Potassium carbonate.
It constitutes 26 percent of Earths mass.
Potassium carbonate is the primary. Energy of first ionisation. Electronic shell Ar 4s 1. To do this he used the relatively new force of. Its specific gravity is 0862 at 20C. The exact reaction however depends on the type of alcohol ie.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It decomposes on boiling. Use your filter properly. Partial Oxidation of Primary Alcohols Reaction. Used to produce Dutch process chocolate by alkalization. The solubility of potassium dichromate in the water at different temperatures is 49 g100 mL at 0 C and 13 g100 mL 20 C and 102 g100 mL at 100 C which means it becomes highly soluble in water at higher temperatures.
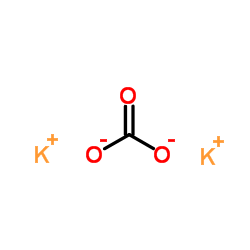 Source: chemsrc.com
Source: chemsrc.com
The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. The vinegar causes the carbonate to be separated from the calcium. Potassium carbonate is the primary. Primary alcohol aldehyde Reagent. Just try soaking a hard boiled egg in vinegar overnight.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Its specific gravity is 0862 at 20C. The solubility of potassium dichromate in the water at different temperatures is 49 g100 mL at 0 C and 13 g100 mL 20 C and 102 g100 mL at 100 C which means it becomes highly soluble in water at higher temperatures. Use a limited amount. Just try soaking a hard boiled egg in vinegar overnight. Food Chemical Codex FCC.
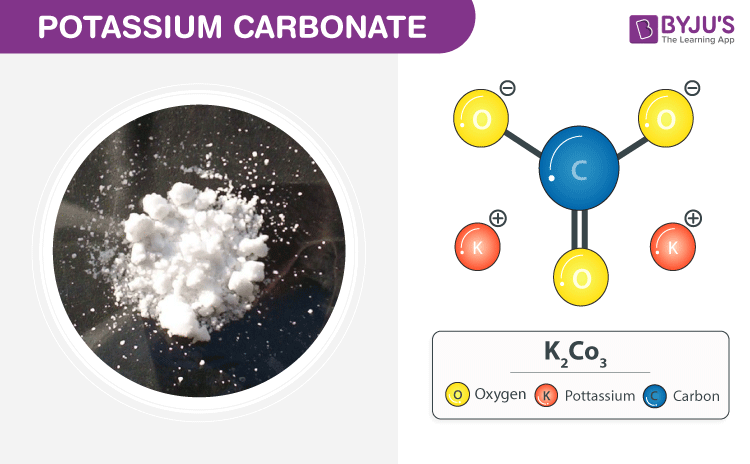 Source: byjus.com
Source: byjus.com
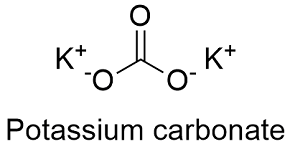
Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. 891 C 1636 F. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. Potassium Carbonate Structure K2CO3. Boiling Point of Potassium carbonate.
Source: chemspider.com
The carbonate evaporates as bubbles and the calcium is left in the vinegar. This will extract all of the calcium from the shell 1 shell is about 2000mg calcium. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. It decomposes on boiling. The carbonate evaporates as bubbles and the calcium is left in the vinegar.
 Source: softschools.com
Source: softschools.com
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. S Density g cm 3. Potassium Carbonate Structure K 2 CO 3. Melting Point of Potassium carbonate. Potassium has two oxidation states 1 and -1rarely.
 Source: sciencemadness.org
Source: sciencemadness.org
The carbonate evaporates as bubbles and the calcium is left in the vinegar. The exact reaction however depends on the type of alcohol ie. Robert makes an excellent point here. Used in the production of wire or. Sediment debris and lead particles can collect in your aerator.
 Source: en.wikipedia.org
Source: en.wikipedia.org
PH 401 500 700 and 1001 buffers are color coded for ease of use. The solubility of potassium dichromate in the water at different temperatures is 49 g100 mL at 0 C and 13 g100 mL 20 C and 102 g100 mL at 100 C which means it becomes highly soluble in water at higher temperatures. Whether it is primary secondary or tertiary and on the conditions. It decomposes on boiling. Potassium carbonate is mainly used in the production of soap and glass.
 Source: researchgate.net
Source: researchgate.net
Potassium Carbonate Anhydrous Certified ACS Revision Date 18-Sep-2018 9. Potassium is the seventh most abundant element found on Earths crust. 671 K while its boiling point is 500 C 932 F. The solubility of potassium dichromate in the water at different temperatures is 49 g100 mL at 0 C and 13 g100 mL 20 C and 102 g100 mL at 100 C which means it becomes highly soluble in water at higher temperatures. Robert makes an excellent point here.
 Source: chemicalbook.com
Source: chemicalbook.com
The carbonate evaporates as bubbles and the calcium is left in the vinegar. The chemical symbol K comes from kalium the Mediaeval Latin for potash which may have derived from the arabic word qali meaning. These chemicals meet the FDA requirements for food use. Potassium Carbonate Structure K2CO3. Whether it is primary secondary or tertiary and on the conditions.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title potassium carbonate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.