Point groups for naphthalene
Home » datasheet » Point groups for naphthalenePoint groups for naphthalene
Point Groups For Naphthalene. We have excluded carboxyl COOH because it does not form when the carbon content exceeds 87. A negative sign is used in this equation to indicate that the freezing point of the solvent decreases when a solute is added. Impurities in a compound can be detected very easily by. Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atomsThe term unsaturated means more hydrogen atoms may be added to the hydrocarbon to make it saturated ie.
 Synchrotron Based Highest Resolution Fourier Transform Infrared Spectroscopy Of Naphthalene C 10 H 8 And Indole C 8 H 7 N And Its Application T Faraday Discussions Rsc Publishing Doi 10 1039 C0fd00013b From pubs.rsc.org
Synchrotron Based Highest Resolution Fourier Transform Infrared Spectroscopy Of Naphthalene C 10 H 8 And Indole C 8 H 7 N And Its Application T Faraday Discussions Rsc Publishing Doi 10 1039 C0fd00013b From pubs.rsc.org
The South African TB Vaccine Initiative SATVI which includes Mark Hatherill Director Tom Scriba Deputy Director and Elisa Nemes. Repel possums by sprinkling the roof cavity with quassia chips from hardware stores or naphthalene or camphor Note. The unknown sample was dissolved in 30mL of. To recrystallize the naphthalene heat about 50 mL of the 31 methanolwater mixture almost to the boiling point on a steam bath see p. IRIS Assessment of Ethyl Tertiary Butyl Ether ETBE - Final. It is the point of maximum energy on the pathway of minimum energy on the landscape from reactants to products.
The configuration of an unsaturated carbons include straight chain such as alkenes and alkynes as well as branched chains and aromatic compounds.
To obtain a more reasonable model and a better understanding of the above. In the 1980s approximately 65 million tonnes of these esters were. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. The purer the sample the narrower the melting point range. Balmer 2 Experimental Procedure Fig1. The naphthalen-2-ol is dissolved in sodium hydroxide solution to produce an ion just like the phenol one.
 Source: youtube.com
Source: youtube.com
218 C LabNetwork old LN00194168. Absorption spectrometry can be utilized to study Cis-Trans isomerism. Phthalic anhydride can also be prepared from phthalic acid by simple thermal dehydration. The primary use of phthalic anhydride is a precursor to phthalate esters used as plasticizers in vinyl chloridePhthalate esters are derived from phthalic anhydride by the alcoholysis reaction. 218 C Alfa Aesar.
 Source: numerade.com
Source: numerade.com
Phthalic anhydride can also be prepared from phthalic acid by simple thermal dehydration. The primary use of phthalic anhydride is a precursor to phthalate esters used as plasticizers in vinyl chloridePhthalate esters are derived from phthalic anhydride by the alcoholysis reaction. To obtain a more reasonable model and a better understanding of the above. Based on the results from. The purer the sample the narrower the melting point range.
 Source: study.com
Source: study.com
Public meetings. For example water H 2 O has a melting point of 4 o C and a boiling point of 100 o C compared with NaCl that has a melting point of 801 o C and a boiling point of 1413 o C. Exposure to large amounts of naphthalene may also cause nausea vomiting diarrhea blood in the urine and a yellow color to the skin. The primary use of phthalic anhydride is a precursor to phthalate esters used as plasticizers in vinyl chloridePhthalate esters are derived from phthalic anhydride by the alcoholysis reaction. Naphthalene has two benzene rings fused together.
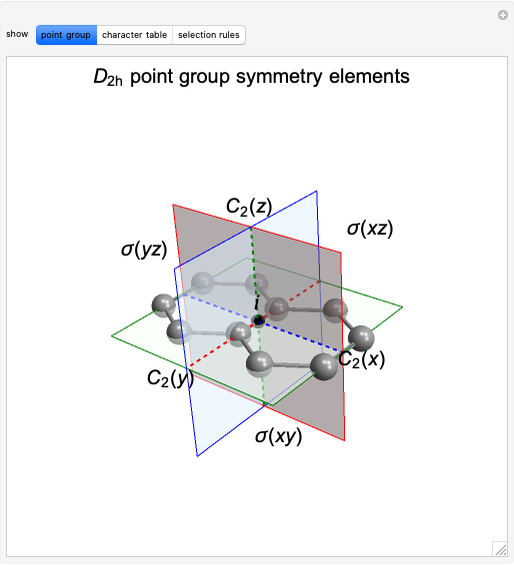 Source: demonstrations.wolfram.com
Source: demonstrations.wolfram.com
They allow the PSM of the SBUs involving covalent interactions such as silylation reactions. The combination of the light and the smell should drive the possum out of your roof and hopefully into the possum house you have provided. Tailor made material to give high electrode density. The purer the sample the narrower the melting point range. The trans-isomer is usually more elongated than its cis counterpart.

Some symptoms of hemolytic anemia are fatigue lack of appetite restlessness and pale skin. Search IRIS By Chemical CASRN or Keyword. We would like to show you a description here but the site wont allow us. Geometrical isomers differ in the spatial arrangement of groups about a plane. It contains an -OH group attached to a naphthalene molecule rather than to a simple benzene ring.
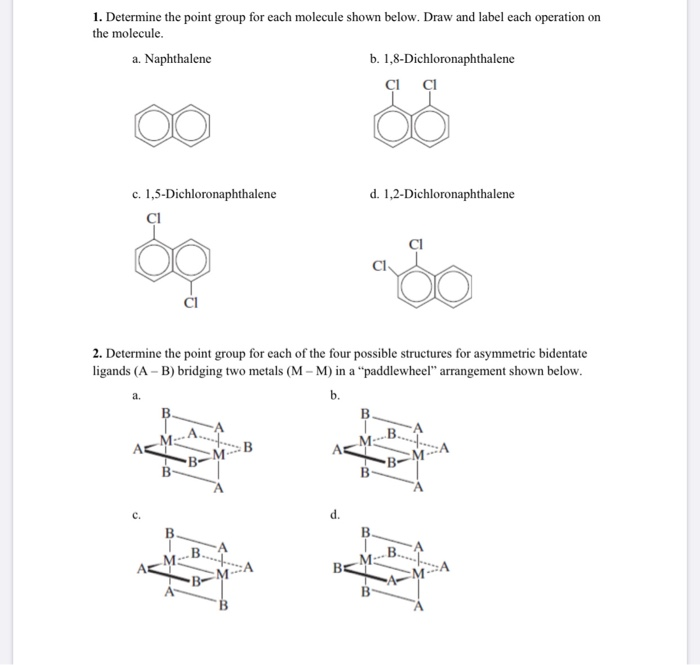 Source: chegg.com
Source: chegg.com
All the absorptions do not shift by the same amount so for anthracene green shaded box and tetracene blue shaded box the weak absorption is obscured by stronger bands that have experienced a greater red shift. This is not. The naphthalen-2-ol is dissolved in sodium hydroxide solution to produce an ion just like the phenol one. The rate of reaction for haloalkanes by the S N 2 mechanism is methyl primary secondary. The combination of the light and the smell should drive the possum out of your roof and hopefully into the possum house you have provided.
 Source: chegg.com
Source: chegg.com
It is the point of maximum energy on the pathway of minimum energy on the landscape from reactants to products. For example when candle wax undergoes combustion the paraffin is vaporized and reacts with oxygen to produce carbon dioxide and water. This condition is called hemolytic anemia. The naphthalen-2-ol is dissolved in sodium hydroxide solution to produce an ion just like the phenol one. Three multi-investigator groups that operate principally in the TBHIV space.
 Source: pubs.rsc.org
Source: pubs.rsc.org
Largest SNF manufacturer in India. 423-425 F 760 mmHg 2172222-2183333 C 760 mmHg Wikidata Q179724 424 F 760 mmHg 2177778 C 760 mmHg Wikidata Q179724. It will dissolve in a hot mixture of 31 methanolwater but is less soluble when this mixture is cold. 218 C LabNetwork old LN00194168. Largest SNF manufacturer in India.
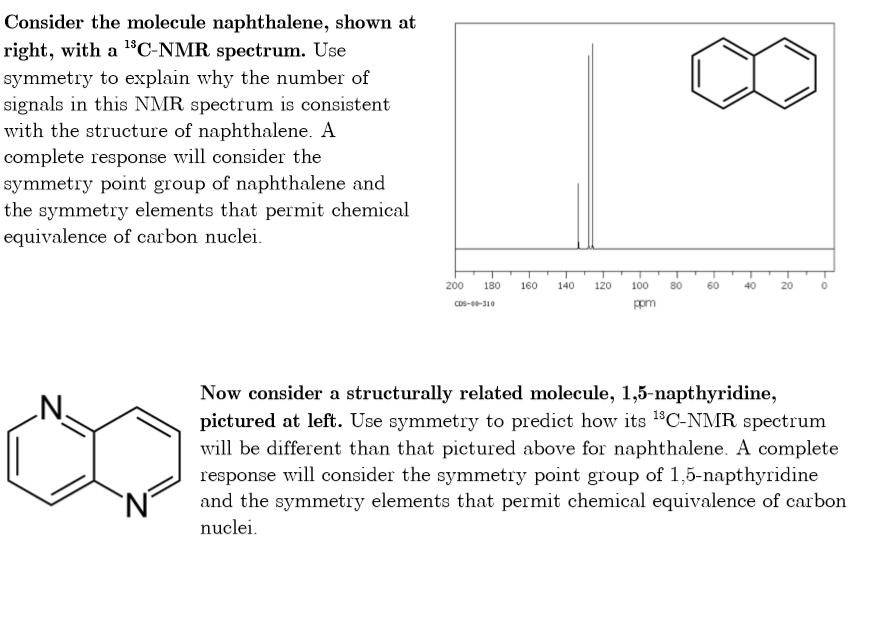 Source: chegg.com
Source: chegg.com
It contains an -OH group attached to a naphthalene molecule rather than to a simple benzene ring. To recrystallize the naphthalene heat about 50 mL of the 31 methanolwater mixture almost to the boiling point on a steam bath see p. The reaction is done under exactly the same conditions as with phenol. Three multi-investigator groups that operate principally in the TBHIV space. Largest SNF manufacturer in India.
 Source: researchgate.net
Source: researchgate.net
Search IRIS By Chemical CASRN or Keyword. The term sublimation only applies to physical changes of state and not to the transformation of a solid into a gas during a chemical reaction. Add some of this hot solvent to. It is the point of maximum energy on the pathway of minimum energy on the landscape from reactants to products. The functional groups in the coal structures include hydroxy OH carboxyl COOH ether oxygen C 2 O.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title point groups for naphthalene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.