Pivalic acid boiling point
Home » datasheet » Pivalic acid boiling pointPivalic acid boiling point
Pivalic Acid Boiling Point. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 407 followed by soaking the herbs n 74 307 and then either using the plant parts intact or as a powder not shown in tableAssessing patient sources of information Table 2 about efficacy of herbs revealed that. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. 1637 C 3267 F.
 Pivalic Acid 99 75 98 9 From sigmaaldrich.com
Pivalic Acid 99 75 98 9 From sigmaaldrich.com
The product is a pivalic acid methyl ester derivative so more Tosylate than other molecule if you see what I mean. The ICSC project is a common undertaking between the World Health Organization WHO and. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 50 0 016 0. When glucose builds up in the blood instead of going into cells the bodys other cells can use this acid for energy and thus continue to starve without insulin. Causes severe skin burns and eye damage Danger Skin corrosionirritationH318 7698.
A The heat input is the same for a reversible and an irreversible process.
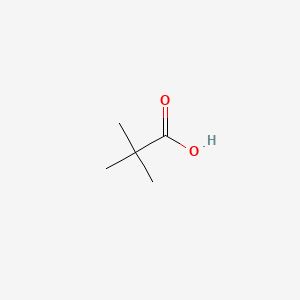
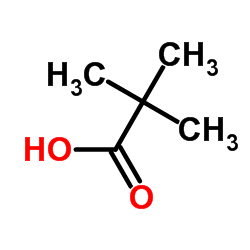
In terms of herb preparation methods boiling the herbs decoction was the most common method of preparation cited by approximately half of the herbal users n 98. Harmful if swallowed Warning Acute toxicity oralH314 100. Pivalic acid is a carboxylic acid with a molecular formula of CH 3 3 CCO 2 H. In terms of herb preparation methods boiling the herbs decoction was the most common method of preparation cited by approximately half of the herbal users n 98. The combination of high excess sugars dehydration and acid build up is known. The combination of high excess sugars dehydration and acid build up is known.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The acid found in vinegar is A. The boiling point of the immiscible liquid system naphthalene water is 98C under a pressure of 733 mmHg. What is Infobox references. So the liver releases the sugar it stores to help out. So the liver releases the sugar it stores to help out.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The product is a pivalic acid methyl ester derivative so more Tosylate than other molecule if you see what I mean. Causes serious eye damage Danger Serious eye. In terms of herb preparation methods boiling the herbs decoction was the most common method of preparation cited by approximately half of the herbal users n 98. The boiling point is 100ºC and the enthalpy of vaporization is 406563 kJ mol1. When glucose builds up in the blood instead of going into cells the bodys other cells can use this acid for energy and thus continue to starve without insulin.

Since the body cannot use these sugars without insulin more sugars piles. The acid found in vinegar is A. The melting point of water at the pressure of interest is 0ºC and the enthalpy of fusion is 60095 kJ mol1. This colourless odiferous organic compound is solid at. So the liver releases the sugar it stores to help out.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Calculate the weight of naphthalene in the distillate. Calculate S for the transformation H2Os 0ºC H2Og 100ºC. The combination of high excess sugars dehydration and acid build up is known. Causes serious eye damage Danger Serious eye. Calculate the weight of naphthalene in the distillate.
Since the body cannot use these sugars without insulin more sugars piles. Compound E chemical formula C5H10O2 is a volatile liquid boiling point 88C. The acid found in vinegar is A. The melting point of water at the pressure of interest is 0ºC and the enthalpy of fusion is 60095 kJ mol1. The combination of high excess sugars dehydration and acid build up is known.
 Source: wikidata.org
Source: wikidata.org
1000 0 100 0. 1000 0 100 0. Pivalic acid is a carboxylic acid with a molecular formula of CH 3 3 CCO 2 H. So the liver releases the sugar it stores to help out. Academiaedu is a platform for academics to share research papers.
 Source: dougdiscovery.com
Source: dougdiscovery.com
Into the blood stream. The combination of high excess sugars dehydration and acid build up is known. The ICSC project is a common undertaking between the World Health Organization WHO and. In terms of herb preparation methods boiling the herbs decoction was the most common method of preparation cited by approximately half of the herbal users n 98. Into the blood stream.

50 0 016 0. Calculate S for the transformation H2Os 0ºC H2Og 100ºC. 1000 0 100 0. Harmful if swallowed Warning Acute toxicity oralH314 100. 407 followed by soaking the herbs n 74 307 and then either using the plant parts intact or as a powder not shown in tableAssessing patient sources of information Table 2 about efficacy of herbs revealed that.
 Source: tcichemicals.com
Source: tcichemicals.com
4368 K Related compounds Related compounds. 1000 0 100 0. The combination of high excess sugars dehydration and acid build up is known. May be corrosive to metals Warning Corrosive to MetalsH302 9964. A colourless viscous water-miscible liquid with a high 210 boiling point it is used in the synthesis of certain polymers and as a solvent and antifreezeIt has a role as a protic solvent and a metabolite.
 Source: chemsrc.com
Source: chemsrc.com
1637 C 3267 F. Since the body cannot use these sugars without insulin more sugars piles. Since the body cannot use these sugars without insulin more sugars piles. What is Infobox references. Pivalic acid is a carboxylic acid with a molecular formula of CH 3 3 CCO 2 H.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title pivalic acid boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.