Physical properties of kmno4 boiling point
Home » datasheet » Physical properties of kmno4 boiling pointPhysical properties of kmno4 boiling point
Physical Properties Of Kmno4 Boiling Point. Information on basic physical and chemical properties Physical state. Uses of Freezing Point Depression. Properties of Transition Metals. Are low energy stable You need energy to.
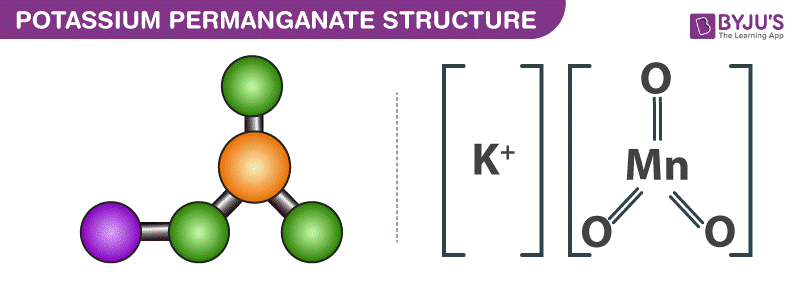 Potassium Permanganate Kmno4 Uses Structure Formula Preparation From byjus.com
Potassium Permanganate Kmno4 Uses Structure Formula Preparation From byjus.com
Oxidation states of transition metals follow the general rules for most other ions except for the fact that the d. Get reaction going like gas bbq How does energy relate to gas bbq. Potassium permanganate occurs in the form of monoclinic prisms almost opaque with a blue metallic lustre. KMnO4 is potassium permanganate where manganese is in the 7 state with no electrons in the 4s and 3d orbitals. If the temperatures are below 18 o C calcium chloride is used instead of NaCl to melt the ice on the roads. Types of solutions expression of concentration of solutions of solids in liquids solubility of gases in liquids solid solutions colligative properties-relative lowering of vapour pressure Raoults law elevation of boiling point depression of freezing point osmotic pressure determination of molecular masses using colligative properties abnormal molecular mass.
Types of solutions expression of concentration of solutions of solids in liquids solubility of gases in liquids solid solutions colligative properties-relative lowering of vapour pressure Raoults law elevation of boiling point depression of freezing point osmotic pressure determination of molecular masses using colligative properties abnormal molecular mass.
Potassium permanganate occurs in the form of monoclinic prisms almost opaque with a blue metallic lustre. Oxidation states of transition metals follow the general rules for most other ions except for the fact that the d. It is soluble in water acetone acetic acid methanol and pyridine. Start w high energy. Are low energy stable You need energy to. KMnO4 is potassium permanganate where manganese is in the 7 state with no electrons in the 4s and 3d orbitals.
Source: chemspider.com
Start w high energy. No data available pH. 16 Melting point. These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons. It is an odourless purple to magenta crystalline solid.
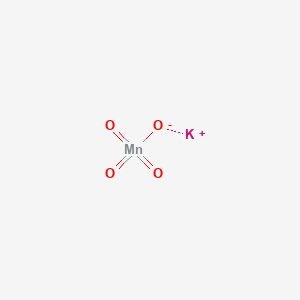
Dark violet-brown Odor. The metals that are found uncombined in nature in large amounts are those that are less. Information on basic physical and chemical properties Physical state. Get reaction going like gas bbq How does energy relate to gas bbq. In fact mercury has a melting point of 3883 C 3789 F and is a liquid at room temperature.
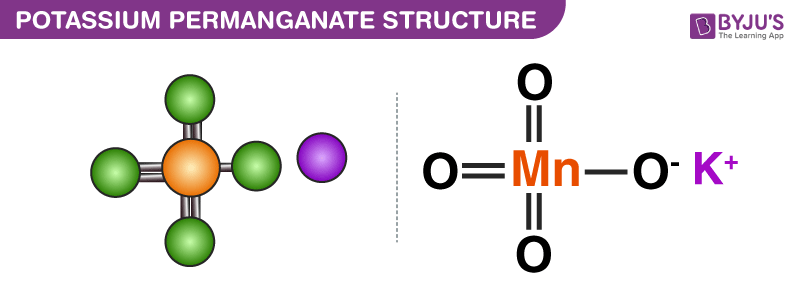 Source: byjus.com
Source: byjus.com
70 - 85 16 pH solution. Wont turn to fire until light it flame you provide is activation energy In a reaction if you. In fact mercury has a melting point of 3883 C 3789 F and is a liquid at room temperature. Physical Properties Of Potassium Permanganate KMnO4. It is soluble in water acetone acetic acid methanol and pyridine.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Dark violet-brown Odor. Properties have to bump into each othercollide at right orientation need exactly right energy Activation energy is like. KMnO4 is potassium permanganate where manganese is in the 7 state with no electrons in the 4s and 3d orbitals. In cold areas where the temperatures range from 0 o C to -15 o C sodium chloride is spread over the roads in order to lower the freezing point of water and prevent the build up of ice. Odorless Odor threshold.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Types of solutions expression of concentration of solutions of solids in liquids solubility of gases in liquids solid solutions colligative properties-relative lowering of vapour pressure Raoults law elevation of boiling point depression of freezing point osmotic pressure determination of molecular masses using colligative properties abnormal molecular mass. Some important uses of freezing point depression are listed below. No data available pH. These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons. The metals that are found uncombined in nature in large amounts are those that are less.
Source: dailymotion.com
It gets dissolved in ethanol and organic solvents. An aqueous solution has a sweetish. Its placement indicates that the metals preceding it will displace it from non-oxidizing acids. Dark violet-brown Odor. Physical Properties Of Potassium Permanganate KMnO4.
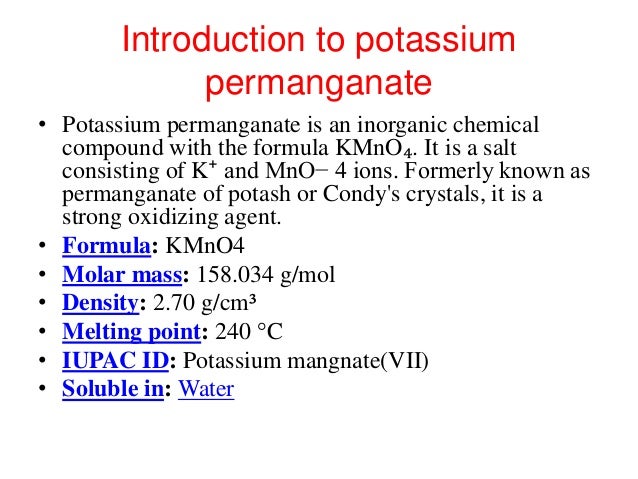 Source: slideshare.net
Source: slideshare.net
These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons. It gets dissolved in ethanol and organic solvents. Get reaction going like gas bbq How does energy relate to gas bbq. These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons. However the Group 12 metals have much lower melting and boiling points since their full d subshells prevent dd bonding.
 Source: britannica.com
Source: britannica.com
CeAr 4s0 3d0nonumber Since the 3p orbitals are all paired this complex is diamagnetic. Properties of Transition Metals. It is soluble in water acetone acetic acid methanol and pyridine. Although hydrogen has many physical and chemical properties that are similar to nonmetals it frequently functions chemically as a metal and for this reason it is included in the activity series of metals. These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons.
 Source: t3db.ca
Source: t3db.ca
If the temperatures are below 18 o C calcium chloride is used instead of NaCl to melt the ice on the roads. KMnO4 is potassium permanganate where manganese is in the 7 state with no electrons in the 4s and 3d orbitals. The metals that are found uncombined in nature in large amounts are those that are less. In cold areas where the temperatures range from 0 o C to -15 o C sodium chloride is spread over the roads in order to lower the freezing point of water and prevent the build up of ice. Odorless Odor threshold.
 Source: byjus.com
Source: byjus.com
Odorless Odor threshold. Oxidation states of transition metals follow the general rules for most other ions except for the fact that the d. Start w high energy. It is an odourless purple to magenta crystalline solid. In cold areas where the temperatures range from 0 o C to -15 o C sodium chloride is spread over the roads in order to lower the freezing point of water and prevent the build up of ice.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title physical properties of kmno4 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.