Phosphoric acid melting point
Home » datasheet » Phosphoric acid melting pointPhosphoric acid melting point
Phosphoric Acid Melting Point. Melting point C phenol. Please Use Our Service If Youre. 393 K decomposes Solubility in water. Acid-base properties of aqueous.
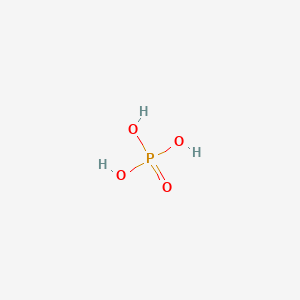
Not less than 195 degC and the determination results in decomposition of aconitic acid. In the control group solely phosphoric acid the mean microTBS was 534-106 MPa which was significantly higher than any hydrofluoric acid prepared group group A versus groups B-E p. Freezing pointContainer materials should be made of stainless steel 316-L high-density polyethylene or. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Arsenic acid as such has not been isolated but is only found in solution where it is largely ionized. Not more than 3 parts per million.
Its hemihydrate form.
PKa is used to describe the acid dissociation. Group B versus group C 135-55 MPa and 187-43 MPa respectively or group D versus group. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. You may examine models of these compounds by clicking on the desired model picture. 108 F NIOSH 2016 Vapor Pressure. See solubility in.
 Source: chemsrc.com
Source: chemsrc.com
2-methylobenzenol o-cresol C 6 H 4 CH 3OH. At the melting point the solid and liquid phases exist in equilibrium. 6 Readily carbonizable substances. 5000 pounds EPA List of Lists 2015 DHS Chemical Facility Anti-Terrorism Standards CFATS No regulatory information available. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials.
Source: chemspider.com
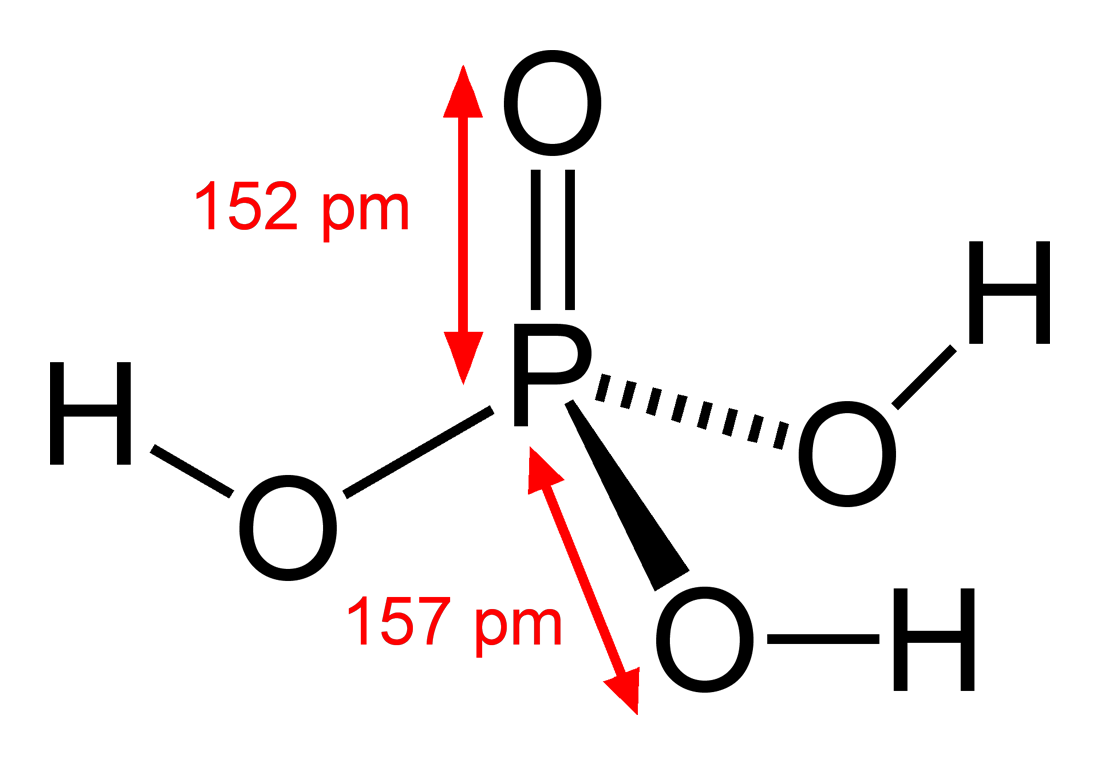
4 Arsenic as As. Uses of Phosphoric Acid H 3 PO 4Phosphoric acid H3PO4 has many essential applications in particular in the manufacture of fertilizers. H 3 PO 4. Contact may severely irritate skin eyes and mucous membranes. This compound is made up of four phosphorus atoms and 10 oxygen atoms bonded together with covalent bonds.

108 F NIOSH 2016 Vapor Pressure. Acidic food and beverage like fruit juice citric acid sparkling drinks carbon dioxide soft drinks phosphoric acid pickles or other acidified food are preserved with benzoic acid and benzoates. OH 3 this colorless acid is the arsenic analogue of phosphoric acid. 03 kPa 20C Vapor Density. Not less than 195 degC and the determination results in decomposition of aconitic acid.
 Source: chemsynthesis.com
Source: chemsynthesis.com
See solubility in. 34 Air 1 Volatility. 003 mm Hg NIOSH 2016 Vapor Density Relative to Air. Density 1651 g cm3. This compound is made up of four phosphorus atoms and 10 oxygen atoms bonded together with covalent bonds.
 Source: hydro-land.com
Source: hydro-land.com
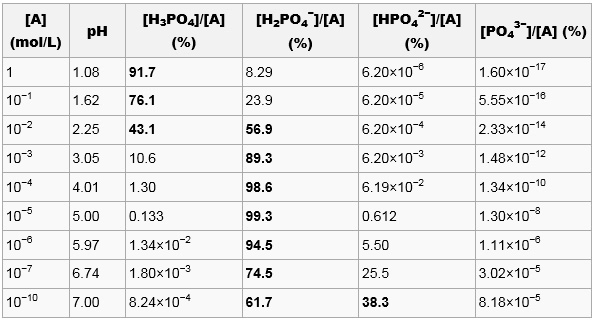
Ionicity in Water. 3086 K Boiling point. The trans-double bond isomer of oleic acid known as elaidic acid has a linear shape and a melting point of 45 ºC 32 ºC higher than its cis isomer. Acid-base properties of aqueous. The melting point is also referred to as liquefaction point solidus or liquidus.
 Source: indiamart.com
Source: indiamart.com
Phosphoric acid is widely used in fertilizers. Group B versus group C 135-55 MPa and 187-43 MPa respectively or group D versus group. The melting point should be recorded as a range the first reading is the temperature at which the sample starts to liquefy and the second reading is taken when the sample is completely melted. The acidity of phosphoric acid may be reduced readily by natural water hardness minerals but the phosphate may persist indefinitely. 122 C 252 F.
 Source: en.wikipedia.org
Source: en.wikipedia.org
We provide solutions to students. Phosphorous acid appears as a white or yellow crystalline solid melting point 701 deg C or a solution of the solid. 4 Arsenic as As. 120 C 248 F. 2 Melting point.
 Source: sciencedirect.com
Source: sciencedirect.com
During transport through the soil phosphoric acid will dissolve some of the soil material in particular carbonate-based materials. 1685 25 C Water 1 Vapor Pressure. 3-methylobenzenol m-cresol C 6 H 4. 122 C 252 F. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure.
 Source: fishersci.se
Source: fishersci.se
It is characteristic of the AT versus GC proportion of the specimen studied due to the fact that there is only 2 hydrogen bounds in AT and 3 in GC a more stable binding. The shapes of stearic and oleic acids are displayed in the models below. Its hemihydrate form. Melting point C phenol. Orthophosphoric acid V H 4 P 2 O 7.
 Source: sciencedirect.com
Source: sciencedirect.com
In the control group solely phosphoric acid the mean microTBS was 534-106 MPa which was significantly higher than any hydrofluoric acid prepared group group A versus groups B-E p. 393 K decomposes Solubility in water. H 3 PO 4. Acidic food and beverage like fruit juice citric acid sparkling drinks carbon dioxide soft drinks phosphoric acid pickles or other acidified food are preserved with benzoic acid and benzoates. 395 K Boiling point.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phosphoric acid melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.