Phenol boiling point
Home » datasheet » Phenol boiling pointPhenol boiling point
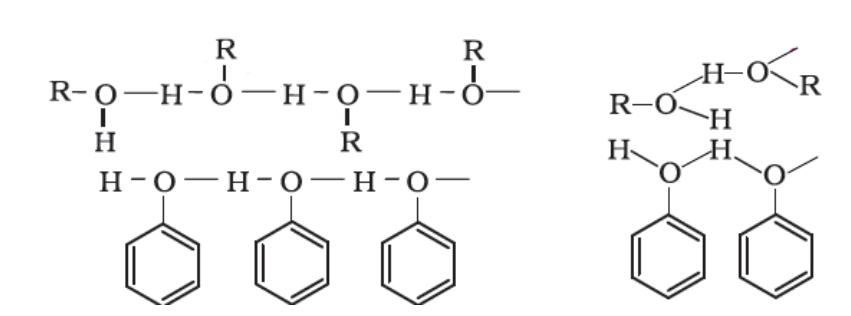
Phenol Boiling Point. Mildly acidic it requires. This is due to the presence of intermolecular hydrogen bonding between hydroxyl groups of phenol molecules. 4-methylobenzenol p-cresol C 6 H 4 CH 3OH. Therefore taking a melting point can aid in identifying an organic compound and assessing its purity.
NTC Thermistors Epoxy. Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source. NTP 1992 Ionization Potential. Therefore taking a melting point can aid in identifying an organic compound and assessing its purity. 462 234 1115. 4-methylobenzenol p-cresol C 6 H 4 CH 3OH.
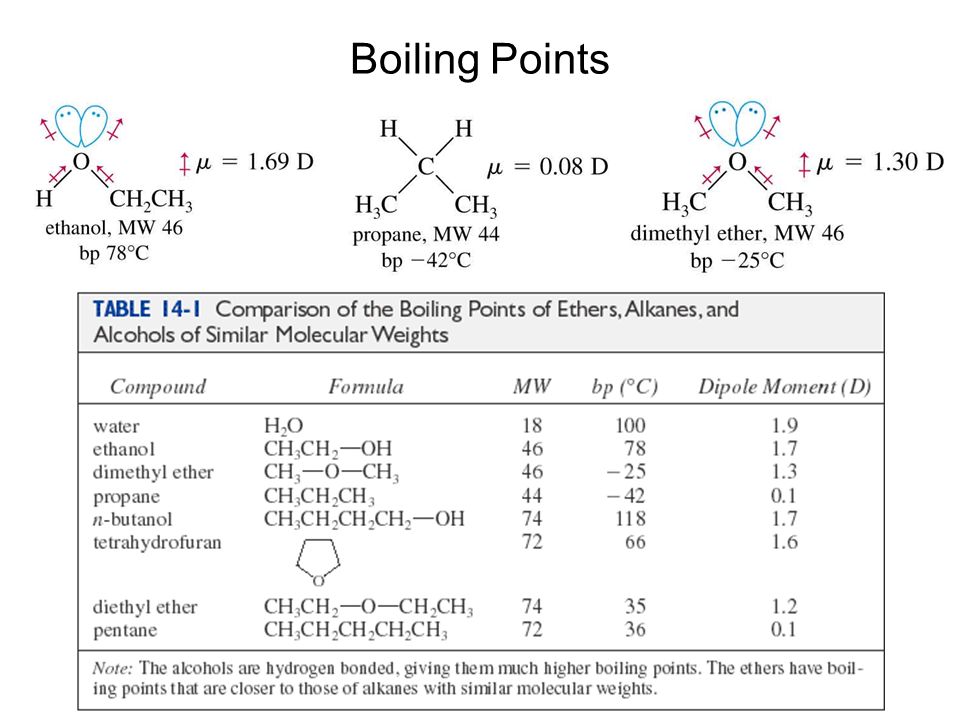
In general the boiling point of phenols increases with an increase in the number of carbon atoms.
Solved Examples Example 1. Potent activator of the human ion channels transient receptor potential V3 TRPV3 and A1 TRPA1. On the basis of this titration dilute the Folin reagent about 2-fold to make it 1 N in acid. 1179 307 166 390 K b K f. Working standards may be prepared from human serum diluted IOO- to lOOO-fold approximately 700 to 70 y per ml. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
 Source: thermopedia.com
Source: thermopedia.com
The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. 801 265 55 512 K b K f. MW 92 bp 111 C 2318 F. ERPGs Emergency Response Planning Guidelines No ERPG information available.
 Source: slideplayer.com
Source: slideplayer.com
Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Emergency eye wash fountains and safety showers should be available in the immediate vicinity of use or handling. The molecule consists of a phenyl group C 6 H 5 bonded to a hydroxy group OH. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. A liquid boils when its vapour pressure is equal to the atmospheric pressure.
 Source: researchgate.net
Source: researchgate.net
Working standards may be prepared from human serum diluted IOO- to lOOO-fold approximately 700 to 70 y per ml. Phenol evaporates more slowly than water and a moderate amount can form a solution with water. 2040 595 179 40 K f. It is a white crystalline solid that is volatile. Combustible liquid refers to any liquid having a flash point at or above 100F and are subdivided as follows.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. Compound like boiling point density and refractive index. C 10 H 7 OH. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 1843 369 596 587 K b K f.
 Source: researchgate.net
Source: researchgate.net
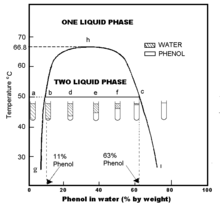
Phenol dissolves to give a 93 percent solution. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. In general the boiling point of phenols increases with an increase in the number of carbon atoms. 1843 369 596 587 K b K f. Emergency eye wash fountains and safety showers should be available in the immediate vicinity of use or handling.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
This is due to the presence of intermolecular hydrogen bonding between hydroxyl groups of phenol molecules. Boiling point helps identify and characterise a compound. Approved flammable storage cabinet is one which has self-closing doors and is in compliance with OSHA 29 CFR 1910106d3 NFPA 30 and UFC 79. Vapour pressure is determined by the kinetic energy of a molecule. 1 kg of the given solution contains 0035kg of NaCl and 0965kg of H.

Phenol also called carbolic acid is an aromatic organic compound with the molecular formula C 6 H 5 OH. 1 kg of the given solution contains 0035kg of NaCl and 0965kg of H. These in turn may be checked against a. Approved flammable storage cabinet is one which has self-closing doors and is in compliance with OSHA 29 CFR 1910106d3 NFPA 30 and UFC 79. Kinetic energy depends on the temperature mass and.

They provide accurate temperature. Phenol dissolves to give a 93 percent solution. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. It is also used in slimicides chemicals that kill bacteria and fungi in slimes as a disinfectant and antiseptic and in medicinal preparations such as.
 Source: byjus.com
Source: byjus.com
In this experiment you will be. Thermometrics NTC Thermistors Epoxy Interchangeable Type 65. Boiling point helps identify and characterise a compound. The ability of phenols to form strong hydrogen bonds also enhances their solubility in water. They provide accurate temperature.
 Source: intechopen.com
Source: intechopen.com
AEGLs Acute Exposure Guideline Levels No AEGL information available. NTC Thermistors Epoxy. C 10 H 7 OH. ACGIH 6153-56-6 Oxalic acid dihydrate TWA 1000000 mgm3 USA. Boiling Point of Phenols.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phenol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.