Palmitic acid melting point
Home » datasheet » Palmitic acid melting pointPalmitic acid melting point
Palmitic Acid Melting Point. So if this is study is right and I see no reason to dismiss it out of hand if you lather your food in palm. As the mechanism or rate of chemical degradation of a drug is likely to be different in solid and liquid state melt of the API or any formulation ingredient should be prevented during an APS study of a solid drug substance or formulation by gathering the melting points of the API. The latter have higher melting points. This observed in the series lauric C12 palmitic C16 stearic C18.
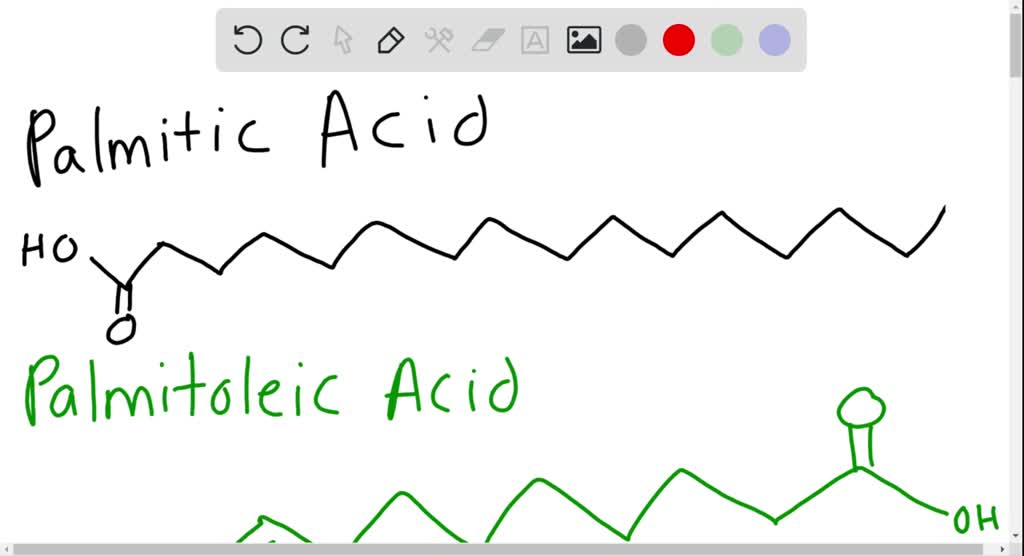 Solved Palmitic Acid And Palmitoleic Acid Both Have 16 Carbons Which Molecule Would Have The Lower Melting Point And Why From numerade.com
Solved Palmitic Acid And Palmitoleic Acid Both Have 16 Carbons Which Molecule Would Have The Lower Melting Point And Why From numerade.com
3360 K Boiling point. Melting point C caprylic octanoic C 7 H 15 COOH 8 0 165 capric decanoic C 9 H 19 COOH 10 0 315 lauric dodecanoic C 11 H 23 COOH. Again changes in crystal packing and intermolecular forces are responsible. In the table of fatty acids we see that the presence of a cis-double bond significantly lowers the melting point of a compound. Unsaturated fatty acids Monosunsaturated. The choice of lauric acid is convenient because the melting point of the pure compound is relatively high 438C.
Similarly oleopalmitostearin C 3 H 5 OCOC 1 5 H 3 1OCOC 1 7 H 3 3OCOC 1 7 H 3 5 contains one radical each of oleic.
Melting point C caprylic octanoic C 7 H 15 COOH 8 0 165 capric decanoic C 9 H 19 COOH 10 0 315 lauric dodecanoic C 11 H 23 COOH. Salts of sodium potassium calcium and magnesium are formed when fatty acids react with these salts. Vegetable-based fatty acids normally consist in. 629 C 1452 F. Citation needed Potential medicinal. A carboxylic acid is a compound that contains a carboxyl group -COOH.
The factors that influence the relative boiling points and water. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. For example the melting point of ice frozen water is 0 C. Fats have the specific gravity less than 1 and also lower than water therefore they float on water. Melting Point C Physical Form.
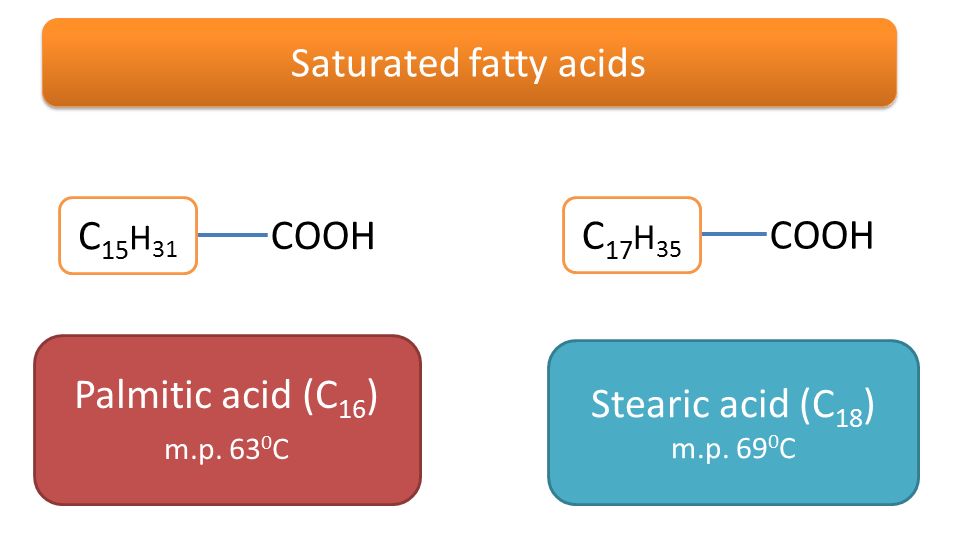
B100 pure biodiesel made from. Palmito-oleic acid is more soluble than palmitic acid. You may examine models of these compounds by clicking on the desired model picture. For example biodiesel made from beef tallow and pork lard has a cloud point in the range of 55F to 60F. Room temperature is 25 o C Lauric acid which melts at 44 o is still a solid while arachidonic acid has long since melted at -50 o.
 Source: fishersci.co.uk
Source: fishersci.co.uk
3360 K Boiling point. Saturated fatty acids such as stearic acid 18 carbons or palmitic acid 16 carbons are solid at room temperature whereas unsaturated fatty acids like linoleic acid 18 carbons with two double bonds at the 9 and 12 positions can be liquid Table 76. It has a role as an EC 3111 carboxylesterase inhibitor an Escherichia coli metabolite a plant metabolite a Daphnia galeata metabolite a solvent an antioxidant and a mouse metabolite. At the melting point the solid and liquid phases exist in equilibrium. Thus palmitoleic acid melts over 60º lower than palmitic acid and similar decreases occur for the C 18 and C 20 compounds.
 Source: numerade.com
Source: numerade.com
Palmitic acid is the first fatty acid produced during fatty acid synthesis and is the precursor to longer fatty acids. Boiling Point C Feature. Dude Palmitic acid is the principal constituent of refined palm oil the stuff is 45-50 palmitic acid and people tend to consume large quantities of palm oil in products where it is usedBy comparison canola oil has only around 5 palmitic acids both are favourites for deep frying. Most abundant is oleic acid 181 Numbering of unsaturated fatty acids starts from the other end of COOH See structural. The exact composition strongly influences cocoa butters melting temperature and chocolate makers sometimes adjust the ratio of these fats in order to fine-tune that melting point.
 Source: scbt.com
Source: scbt.com
Again changes in crystal packing and intermolecular forces are responsible. Similarly oleopalmitostearin C 3 H 5 OCOC 1 5 H 3 1OCOC 1 7 H 3 3OCOC 1 7 H 3 5 contains one radical each of oleic. The shapes of stearic and oleic acids are displayed in the models below. With a typical melting point ranging from 88F-95F it is a stiff solid at room temperature. Oleic acid is an octadec-9-enoic acid in which the double bond at C-9 has Z cis stereochemistry.

It is also a temperature at which a solid crystal turns into a liquid. Dude Palmitic acid is the principal constituent of refined palm oil the stuff is 45-50 palmitic acid and people tend to consume large quantities of palm oil in products where it is usedBy comparison canola oil has only around 5 palmitic acids both are favourites for deep frying. Melting Point C Physical Form. Salts of sodium potassium calcium and magnesium are formed when fatty acids react with these salts. Saturated fatty acids such as stearic acid 18 carbons or palmitic acid 16 carbons are solid at room temperature whereas unsaturated fatty acids like linoleic acid 18 carbons with two double bonds at the 9 and 12 positions can be liquid Table 76.

Thus stearodipalmitin C 3 H 5 OCOC 1 5 H 3 1 2 OCOC 1 7 H 3 5 contains two palmitic acid radicals and one stearic acid radical. Unsaturated fatty acids Monosunsaturated. As the mechanism or rate of chemical degradation of a drug is likely to be different in solid and liquid state melt of the API or any formulation ingredient should be prevented during an APS study of a solid drug substance or formulation by gathering the melting points of the API. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. In addition the melting point of a fatty acid depends on whether the chain is even- or odd-numbered.
 Source: tcichemicals.com
Source: tcichemicals.com
Thus stearodipalmitin C 3 H 5 OCOC 1 5 H 3 1 2 OCOC 1 7 H 3 5 contains two palmitic acid radicals and one stearic acid radical. Again changes in crystal packing and intermolecular forces are responsible. This product is free of the 8 major allergens. The choice of lauric acid is convenient because the melting point of the pure compound is relatively high 438C. The melting point of fats depends upon their constituent fatty acids.
 Source: researchgate.net
Source: researchgate.net
The melting point is specific for a given substance. The melting point of fats depends upon their constituent fatty acids. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Fatty acid products are normally mixtures of several fatty acids with different structures. Melting Point C Physical Form.
 Source: researchgate.net
Source: researchgate.net
Saturated fatty acids such as stearic acid 18 carbons or palmitic acid 16 carbons are solid at room temperature whereas unsaturated fatty acids like linoleic acid 18 carbons with two double bonds at the 9 and 12 positions can be liquid Table 76. The factors that influence the relative boiling points and water. Fatty Acid Content of Animal Fats. Salts of sodium potassium calcium and magnesium are formed when fatty acids react with these salts. It has a role as an EC 3111 carboxylesterase inhibitor an Escherichia coli metabolite a plant metabolite a Daphnia galeata metabolite a solvent an antioxidant and a mouse metabolite.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title palmitic acid melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.