Octane normal boiling point
Home » datasheet » Octane normal boiling pointOctane normal boiling point
Octane Normal Boiling Point. A mixture of different compounds boils over a certain range of temperature reflecting the boiling point of each specific compound present in the mixture. This means you have a problem if you try to use pure isobutane when the temperature drops below -1175C. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment.
 4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
Alkanes are chemical compounds that consist only of the elements carbon C and hydrogen H in proportions according to the general formula. Class IC Liquid Flammable Vapor PEL Flash Boiling Point Point o F o F Limits Density ppm Air 1 Common Name Other Names LEL UEL Isoamyl Acetate. First-Aid Measures FIRST AID -EYE CONTACTImmediately flush eyes with plenty of water for at least 15 minutes holding eyelids open. When used as a test fuel component in anti-knock test engines a 100 heptane fuel is the zero point of the octane rating scale the 100 point is 100 iso-octaneOctane number equates to the anti-knock qualities of a comparison. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. The boiling point depends on the pressure.
Turning from Liquid to Gas.
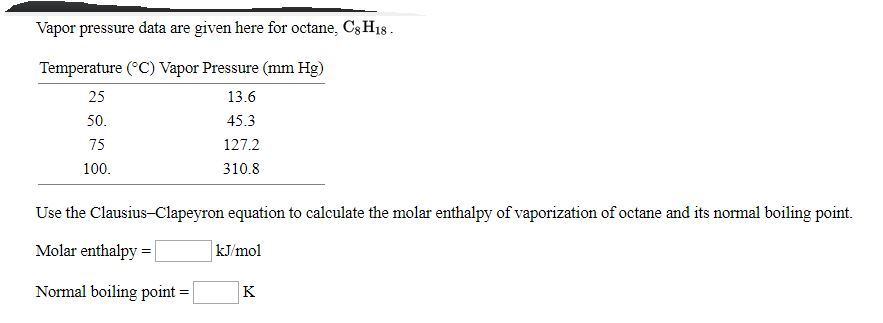
Turning from Liquid to Gas. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. At 40 C the vapor pressure of heptane is 915 torr and the vapor pressure of octane is 312 torr. Boiling and freezing temperatures of selected compounds of diesel fuel are listed in Table 2. Boiling Points of Alkanes Reminder about Alkanes. You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids.
 Source: chemsynthesis.com
Source: chemsynthesis.com
In a column for the fractional distillation of crude oil oil heated to about 425 C in the furnace vaporizes when it enters the base of the tower. The atoms that form alkanes are linked exclusively by single bonds hence alkanes are saturated hydrocarbons. Lets compute the massic volume of liquid at 1bar 1e5 Pa of pressure vL 1 CP. For example the boiling point of water is 100 C. Exponential temperature variation fit.
 Source: chegg.com
Source: chegg.com
PropsSI D P 1e5 Q 0 fluid Same for saturated vapor vG. The S-8 and Sasol GTL-2 fuels are distilled to have boiling point slopes that are typical of conventional jet fuel while the rest are relatively flat. You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids. C 9 H 20. T he temperature at which a liquid turns into a gas.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
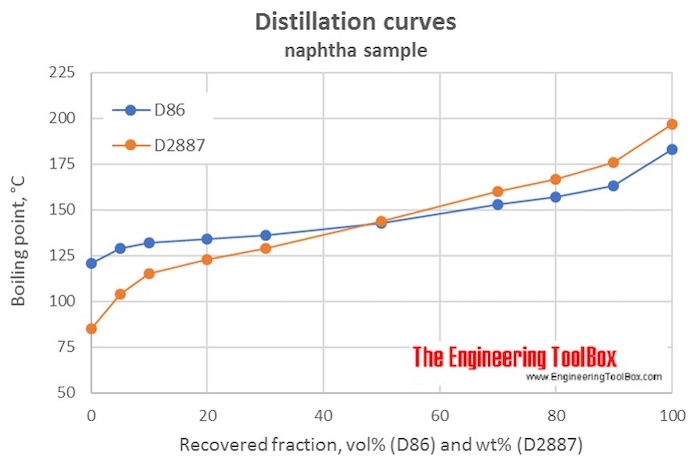
To develop a reliable and general model involved data in development should be various and cover a wide range of conditions. You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids. Exponential temperature variation fit. Shell GTL has narrower range of boiling point. Then the boiling point of each compound is weighted with regard to the fraction.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Turning from Liquid to Gas. Heptane or n-heptane is the straight-chain alkane with the chemical formula H 3 CCH 2 5 CH 3 or C 7 H 16 and is one of the main components of gasoline petrol. Then the boiling point of each compound is weighted with regard to the fraction. This study has included a large databank of 2143 data points for pure normal alkanes and 985 data points for binary mixtures of normal alkanes in a wide range of temperature and pressure from 27815 to 52255 K and 01 MPa27596 MPa respectively. Heptane and octane form an ideal solution.
 Source: chem.libretexts.org
Source: chem.libretexts.org
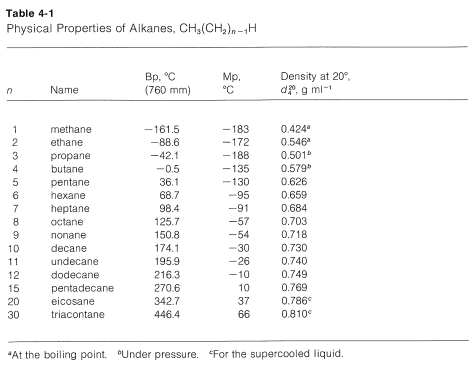
C n H 2n2 where the letter n represents the number of carbon atoms in each molecule. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. For compounds with the same carbon number the order of increasing boiling point by class is isoparaffin n-paraffin naphthene and aromatic. Boiling and freezing point properties Boiling Data at l atm Freezing Data Liquid Properties Normal Latent Heat of Latent Heat Specific Boiling Vaporization Freezing of Fusion Temperature Density Heat Substance Point C h fg kJkg Point C h if kJkg C r kgm3 c p kJkgK Ammonia 333 1357 777 3224 333 682 443 20 665 452 0 639 460 25 602 480 Argon 1859 1616 1893 28 1856 1394 1. A mixture of different compounds boils over a certain range of temperature reflecting the boiling point of each specific compound present in the mixture.
 Source: chegg.com
Source: chegg.com
C 9 H 20. For compounds with the same carbon number the order of increasing boiling point by class is isoparaffin n-paraffin naphthene and aromatic. - vapours start to decompose at about 900 K. When used as a test fuel component in anti-knock test engines a 100 heptane fuel is the zero point of the octane rating scale the 100 point is 100 iso-octaneOctane number equates to the anti-knock qualities of a comparison. Boiling Points of Alkanes Reminder about Alkanes.
 Source: clutchprep.com
Source: clutchprep.com
Isobutane boils at -1175C whereas butane boils at -04C. For compounds of the same class the boiling temperature increases with carbon number. The boiling point is specific for the given substance. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. To develop a reliable and general model involved data in development should be various and cover a wide range of conditions.
 Source: chegg.com
Source: chegg.com
The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. National Toxicology Program Chemical Repository Database. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. Coefficents calculated by NIST from authors data. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F.
 Source: thermopedia.com
Source: thermopedia.com
Then we say that liquid boils. The boiling point distribution curve is noticeably different among the fuels. Then we say that liquid boils. PropsSI D P 1e5 Q 0 fluid Same for saturated vapor vG. Viscosity510-62010-6 m2s at 20 ºC.
 Source: en.wikipedia.org
Source: en.wikipedia.org
To develop a reliable and general model involved data in development should be various and cover a wide range of conditions. The S-8 and Sasol GTL-2 fuels are distilled to have boiling point slopes that are typical of conventional jet fuel while the rest are relatively flat. Generally flash point increases with an increase in boiling point. Temperature K A B C Reference Comment. The anti-knock rating of this mixture would be the same as the percentage of iso octane in the mix.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title octane normal boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.