Octane melting point
Home » datasheet » Octane melting pointOctane melting point
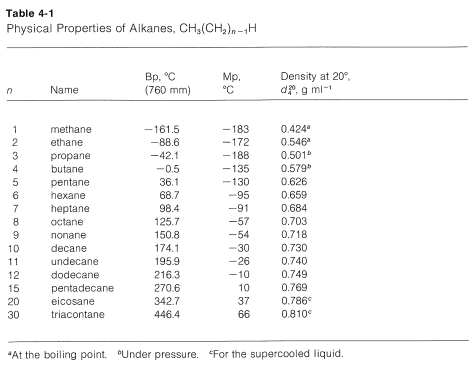
Octane Melting Point. If the length is more the boiling melting point will be higher. The melting and freezing point changes with pressure but normally they are given at 1 atm. Boiling point of water. As is the case with other hydrocarbons octane undergoes hydrocarbon combustion combining with oxygen to form carbon dioxide.
 4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
The melting point depends on the pressure. -269 C -452 F. Alkanes consist of four carbon atoms at standard temperatures. The melting and freezing point changes with pressure but normally they are given at 1 atm. C 8 H 18. C 2 H 6-183-89.
The melting and freezing point changes with pressure but normally they are given at 1 atm.
42 188 201 gas 1 Butane. As the mechanism or rate of chemical degradation of a drug is likely to be different in solid and liquid state melt of the API or any formulation ingredient should be prevented during an APS study of a. As is the case with other hydrocarbons octane undergoes hydrocarbon combustion combining with oxygen to form carbon dioxide. Melting Properties at 300 K kWmKc pJkgK Point r c p k a 106 Composition K kgm3 JkgK WmK m2s 100 200 400 600 800 1000 Aluminum. The hydrocarbon combustion reaction releases heat energy and is. 69 95 659 liquid 5 Heptane.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
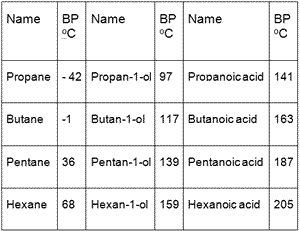
The boiling and melting point of alkane depends upon the length of the carbon chain. If we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen. 56 C 1328 F Boiling point of alcohol. CH 4-162 182 0656 gas 1 Ethane. Cloud point CP is the temperature at which wax first becomes visible when the fuel is cooled ASTM D2500 Pour point is the temperature at which the amount of wax out of solution is sufficient to gel the fuel ASTM D97.
 Source: researchgate.net
Source: researchgate.net
Hydrogen bonding is a special type of chemical bond that involves dipole-dipole attraction between two or more dipolar molecules which are also referred to simply as dipoles. C 10 H 22-30. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale. Causes serious eye irritation Warning Serious eye damageeye irritationH400 9649. For mixtures of compounds as.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Topping our list of the best guitar solos David Gilmours fretwork on Comfortably Numb provides a high point on Pink Floyds 1980 album The Wall. We say that such a body melts. C 9 H 20-51. Melting Point o C Boiling Point o C State at 25 o C. Melting point C Density kgm 3 at 20 C Isomers.
 Source: researchgate.net
Source: researchgate.net
Melting point - the temperature at which a solid turns into a liquid. If the length is more the boiling melting point will be higher. If we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale. Hydrogen Bonds Are Dipole-Dipole Attraction.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Fenghe Qiu in Accelerated Predictive Stability 2018. C 5 H 12-130. C 4 H 10. 7837 C 1731 F Boiling point of nitrogen. C 2 H 6-183-89.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The last compound an isomer of octane is nearly spherical and has an exceptionally high melting point only 6º below the boiling point. Boiling point of water. C 6 H 14-95. For example the melting point of ice frozen water is 0 C. A rousing rock ballad the song is elevated by Gilmours goosebumps-inducing solo a hugely emotive and undeniably vital performance which ebbs and flows with woozy finesse.
 Source: thermopedia.com
Source: thermopedia.com
2C 8 H 18 25O 2 16CO 2 18H 2 O Heat Energy. Melting point - the temperature at which a solid turns into a liquid. It is also a temperature at which a solid crystal turns into a liquid. C 6 H 14. 126 57 703 liquid.
 Source: youtube.com
Source: youtube.com
42 188 201 gas 1 Butane. C 3 H 8. A dipole is a molecule that is electrically neutral. Order now to receive when in stock. Very toxic to aquatic life with long lasting effects Warning Hazardous to the aquatic.
 Source: chem.libretexts.org
Source: chem.libretexts.org
69 95 659 liquid 5 Heptane. The boiling and melting point of alkane depends upon the length of the carbon chain. Melting Properties at 300 K kWmKc pJkgK Point r c p k a 106 Composition K kgm3 JkgK WmK m2s 100 200 400 600 800 1000 Aluminum. Order now to receive when in stock. C 11 H 24-25.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Pure 933 2702 903 237 971 302 237 240 231 218 482 798 949 1033 1146 Alloy 2024-T6 775 2770 875 177 730 65 163 186 186 45 Cu 15 Mg 06 Mn 473 787 925 1042 Alloy 195 Cast 45 Cu 2790 883. C 5 H 12-130. For mixtures of compounds as. 0 138 248 gas 2 Pentane. C 4 H 10-138-05.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title octane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.