Nitroglycerin melting point
Home » datasheet » Nitroglycerin melting pointNitroglycerin melting point
Nitroglycerin Melting Point. An acoustic stringed musical instrument. A usually round flattened base of leavened wheat-based dough topped with tomatoes cheese and often various other ingredients. It acts as a prodrug releasing nitric oxide to open blood vessels and so alleviate heart pain. Insoluble in volatile oils and fixed oils in water it is miscible.
 Nitroglycerin Wikipedia From en.wikipedia.org
Nitroglycerin Wikipedia From en.wikipedia.org
Low molecular weight less than 1000 Daltons Adequate solubility in oil and water. PKa of maleic acid. In the labelling making available on the market and advertising of cosmetic products. Low melting point less than 200 Potent dose ideally less than 10 mg per day. If the transition went from the liquid to the solid state the numerical value for would be the same but the sign would be reversed since we are going from a less ordered to a more ordered situation. The organic nitrates are vasodilators active on both arteries and veins.
Physicochemical properties- It is generally accepted that the best drug candidates for passive adhesive Transdermal patches must be Non-ionic.
9712 orders delivered before the deadline. In the labelling making available on the market and advertising of cosmetic products. We would like to show you a description here but the site wont allow us. Nitroglycerin is dangerously unstable but when mixed with kieselguhr diatomaceous earth which acts as an adsorbent it is safer to handle and does not explode until set off by a smaller explosion from a detonator such as a blasting cap. A Copper and nitric acid. An acoustic stringed musical instrument.
Source: chemspider.com
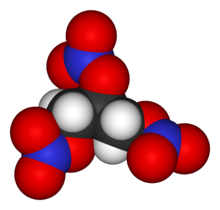
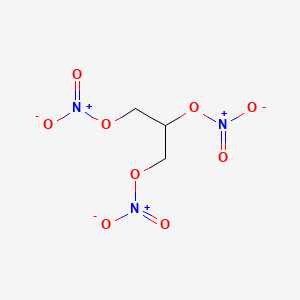
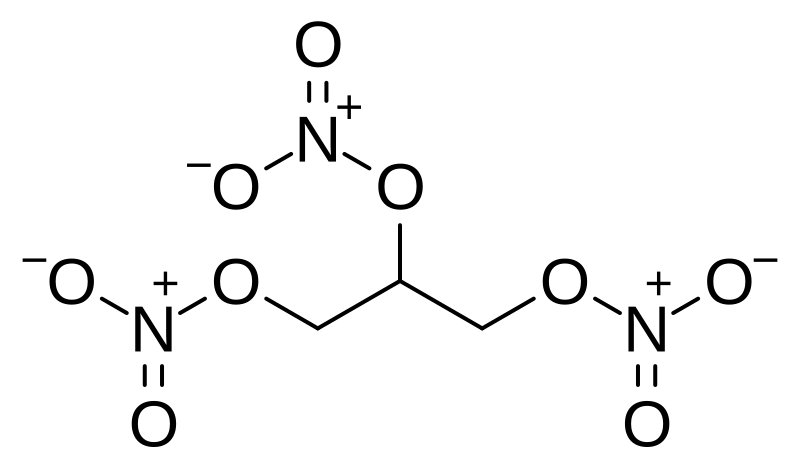
Nitroglycerin NG also known as nitroglycerine trinitroglycerin TNG nitro glyceryl trinitrate GTN or 123-trinitroxypropane is a dense colorless oily explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid esterChemically the substance is an organic nitrate compound rather than. V net v 1 v 0 - 1 1 V net expansion volume of water ft 3 m 3 v 0 specific volume. C 3 H 7 OH-1147. Nitroglycerin is a vasodilator drug used for the treatment of chest pain and high blood pressure. Melting point C ethanol.
To achieve this phase-change a certain amount of heat must be added which goes entirely into changing the phase without raising the temperature. 85 10 average quality score from customers. Like many nitrate esters ETN acts as a vasodilator and was the active ingredient in the original sustained release tablets made under a process patent in the early 1950s called nitroglyn. C 4 H 9 OH-108. The entropy change is positive as the solid state changes into the liquid state.
 Source: acs.org
Source: acs.org
PKa of free base. While there are classic examples such as nitroglycerin which do display a short time for maximum plasma concentration. Dynamite was invented by the Swedish chemist Alfred Nobel in 1867. Other examples of chemical changes include reactions that are performed in a lab such as copper reacting with nitric acid all forms of combustion burning and food being cooked digested or rotting. 9712 orders delivered before the deadline.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Solubility in water Room temperature. The freezing point is lower than the melting point in the case of mixtures and for certain organic compounds such as fatsAs a mixture freezes the solid that forms first usually has a composition different from that of the liquid and formation of the. Double and single spacing. And whose molecular weight is 23614. Low melting point Binary fluorides are unusually volatile compared with the corresponding halides or oxides Reactions.
Source: en.wikipedia.org
123 mgm 2 s. C 4 H 9 OH-108. Elemental nitrogen is nonpolar and has a very low boiling point 774K -1958. The freezing point is lower than the melting point in the case of mixtures and for certain organic compounds such as fatsAs a mixture freezes the solid that forms first usually has a composition different from that of the liquid and formation of the. Insoluble in volatile oils and fixed oils in water it is miscible.
If the transition went from the liquid to the solid state the numerical value for would be the same but the sign would be reversed since we are going from a less ordered to a more ordered situation. V net v 1 v 0 - 1 1 V net expansion volume of water ft 3 m 3 v 0 specific volume. ISMN is freely soluble in water ethanol methanol chloroform ethyl acetate and dichloromethane. Propan-2-ol isopropyl alcohol C 3 H 7 OH-895. 290 degree Celsius melting point.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Insoluble in volatile oils and fixed oils in water it is miscible. Freezing point temperature at which a liquid becomes a solidAs with the melting point increased pressure usually raises the freezing point. Physicochemical properties- It is generally accepted that the best drug candidates for passive adhesive Transdermal patches must be Non-ionic. Propan-2-ol isopropyl alcohol C 3 H 7 OH-895. Low melting point Binary fluorides are unusually volatile compared with the corresponding halides or oxides Reactions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The entropy change is positive as the solid state changes into the liquid state. Other examples of chemical changes include reactions that are performed in a lab such as copper reacting with nitric acid all forms of combustion burning and food being cooked digested or rotting Figure 3. We would like to show you a description here but the site wont allow us. Net expansion volume of water when heated can be expressed as. 1215 Nitroglycerin is available in various forms including a spray form sublingual tablet form intravenous form extended-release tablet form and transdermal form.
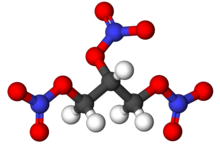 Source: en.wikipedia.org
Source: en.wikipedia.org
Isosorbide dinitrate is a white crystalline odorless compound which is stable in air and in solution has a melting point of 70C and has an optical rotation of 134 c10 alcohol 20C. Nitroglycerin is a vasodilator drug used for the treatment of chest pain and high blood pressure. We would like to show you a description here but the site wont allow us. 9712 orders delivered before the deadline. Intrinsic dissolution rate in water.
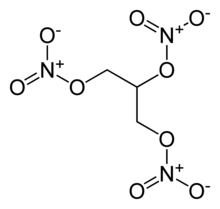 Source: newworldencyclopedia.org
Source: newworldencyclopedia.org
PKa of free base. Double and single spacing. Solubility in saliva room temperature 3 mgmL. Nitroglycerin is dangerously unstable but when mixed with kieselguhr diatomaceous earth which acts as an adsorbent it is safer to handle and does not explode until set off by a smaller explosion from a detonator such as a blasting cap. All this while still possessing tribalistic characteristics such as a huntergatherer style of living.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nitroglycerin melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.