Napthalene point group
Home » datasheet » Napthalene point groupNapthalene point group
Napthalene Point Group. B Label points A-H as either efficient inefficient or unattainable. 2-Naphthol is a widely. The apparent enthalpy of fusion is different and. Investigate how students could detect that a gas is being released from both of these solid substances.
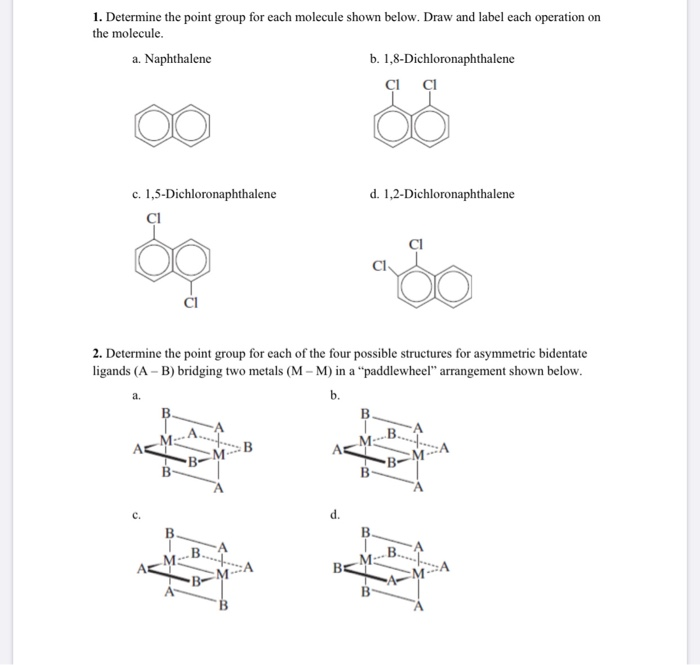 Solved 1 Determine The Point Group For Each Molecule Shown Chegg Com From chegg.com
Solved 1 Determine The Point Group For Each Molecule Shown Chegg Com From chegg.com
The added conjugation in naphthalene anthracene and tetracene causes bathochromic shifts of these absorption bands as displayed in the chart on the left below. 2-Naphthol is a widely. 1964 studied the pyrolysis of naphthalene at 700 C. However modifying a section of the inner wall alters the performance of the integrating sphere. The naphthols are naphthalene homologues of phenol but more reactiveBoth isomers are soluble in simple alcohols ethers and chloroform. If so is it productively efficient.
The freezing point of benzene is 55 C and K f 512 kgmol.
Use examples of dry ice and naphthalene in a Predict-Observe-Explain POE demonstration showing that some substances can change state from a solid to a gas without becoming a liquid. Results are shown for the dissolution of naphthalene in several solvents below. See also 624 635 and 651. There are two effects when the difference between solute and solvent increases. From the point of view of harnessing the potential of artificial molecular machines in devices this is an important observation. To help put these lists into context some related information is included on how different agencies and groups test and classify possible carcinogens.
 Source: chegg.com
Source: chegg.com
This page provides lists of substances and exposures that are known or suspected to cause cancer. However modifying a section of the inner wall alters the performance of the integrating sphere. Use examples of dry ice and naphthalene in a Predict-Observe-Explain POE demonstration showing that some substances can change state from a solid to a gas without becoming a liquid. There are two effects when the difference between solute and solvent increases. B Label points A-H as either efficient inefficient or unattainable.
 Source: pubs.rsc.org
Source: pubs.rsc.org
B Label points A-H as either efficient inefficient or unattainable. Spoon several dry ice pellets into an empty balloon. A 160 g sample of napthalene a non-electrolyte with a formula of C 10 H 8 is dissolved in 200 g of benzene. The slope of the plot changes ie. The added conjugation in naphthalene anthracene and tetracene causes bathochromic shifts of these absorption bands as displayed in the chart on the left below.
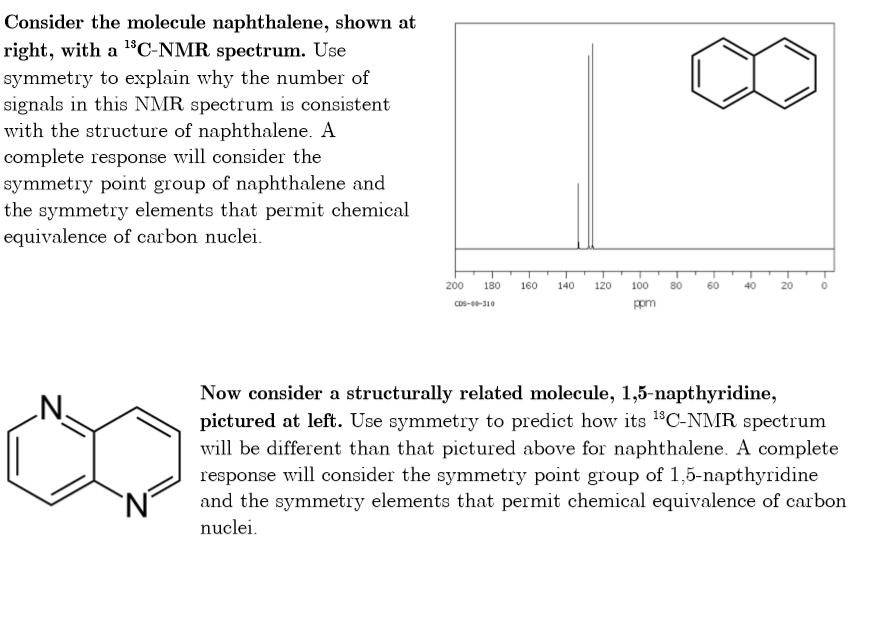 Source: chegg.com
Source: chegg.com
The slope of the plot changes ie. Often a section of the spheres inner wall is replaced by the sample when its transmittance and diffuse reflectance spectra are measured Figure 2. Use examples of dry ice and naphthalene in a Predict-Observe-Explain POE demonstration showing that some substances can change state from a solid to a gas without becoming a liquid. 1964 studied the pyrolysis of naphthalene at 700 C. Do not use a combination of products and putting a light into the roof cavity and leaving it on for three days and nights.

A At point F how many sweaters are being produced. All the absorptions do not shift by the same amount so for anthracene green shaded box and. 042 gL 20 C Acidity pK a 424. The apparent enthalpy of fusion is different and. The slope of the plot changes ie.
 Source: youtube.com
Source: youtube.com
A 160 g sample of napthalene a non-electrolyte with a formula of C 10 H 8 is dissolved in 200 g of benzene. The apparent enthalpy of fusion is different and. An integrating spheres optical performance depends on the reflectance at each point on its entire inner surface. 2-Naphthol is a widely. Results are shown for the dissolution of naphthalene in several solvents below.
 Source: study.com
Source: study.com
C Can we determine if point F is productively efficient. It is an isomer of 1-naphthol differing by the location of the hydroxyl group on the naphthalene ring. Results are shown for the dissolution of naphthalene in several solvents below. Students can detect the naphthalene gas by smell. D Can we determine if point F.
 Source: researchgate.net
Source: researchgate.net
Students can detect the naphthalene gas by smell. The extremely efficient near. Often a section of the spheres inner wall is replaced by the sample when its transmittance and diffuse reflectance spectra are measured Figure 2. Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. The freezing point of benzene is 55 C and K f 512 kgmol.
 Source: numerade.com
Source: numerade.com
The apparent enthalpy of fusion is different and. 042 gL 20 C Acidity pK a 424. However modifying a section of the inner wall alters the performance of the integrating sphere. To help put these lists into context some related information is included on how different agencies and groups test and classify possible carcinogens. The Stoddart group reports in Angewandte Chemie the preparation and properties of single-walled carbon nanotubes wrapped with conducting polymers.
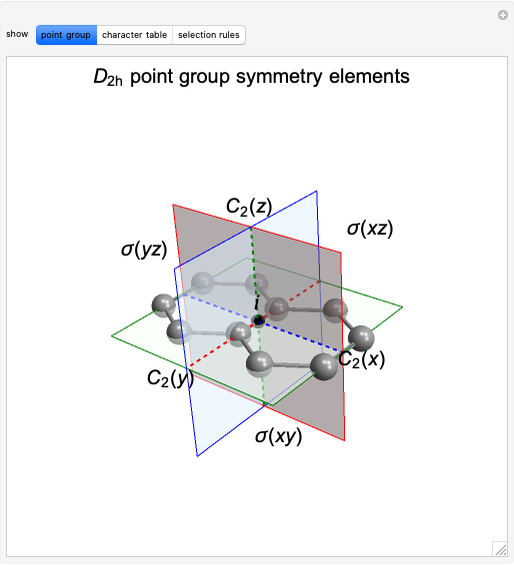 Source: demonstrations.wolfram.com
Source: demonstrations.wolfram.com
To help put these lists into context some related information is included on how different agencies and groups test and classify possible carcinogens. However modifying a section of the inner wall alters the performance of the integrating sphere. For any matters related to purchase of WHO publications please use our WHO Press order form. The added conjugation in naphthalene anthracene and tetracene causes bathochromic shifts of these absorption bands as displayed in the chart on the left below. Results are shown for the dissolution of naphthalene in several solvents below.
 Source: researchgate.net
Source: researchgate.net
How many hard drives. The freezing point of benzene is 55 C and K f 512 kgmol. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. If we take this value to represent the energy cost of introducing one double bond into a six-carbon ring we would expect a cyclohexadiene to release 572 kcal per mole on complete hydrogenation and 135. How many hard drives.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title napthalene point group by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.