Napthalene melting point
Home » datasheet » Napthalene melting pointNapthalene melting point
Napthalene Melting Point. Napthalene plant assets. Methane CH 4-182-164. 1-2-Naphthylethanone beta -methyl naphthyl ketone. Online API to Specific Gravity calculator.

5260 C 9788 F Evaporation rate. -2C 28F Melting Point. Strong Acids Strong oxidizers. The flat shape of aromatic compounds such as napthalene and biphenyl allows them to stack together efficiently and thus aromatics tend to have higher melting points compared to alkanes or alkenes with similar molecular weights. The low melting and boiling points of covalent compounds can be explained as below. Pay careful attention to the health and.
For these cases Whitson correlated the Watson Characterization Factor with the.
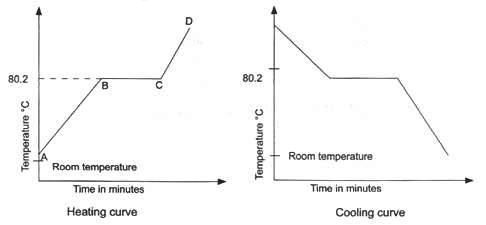
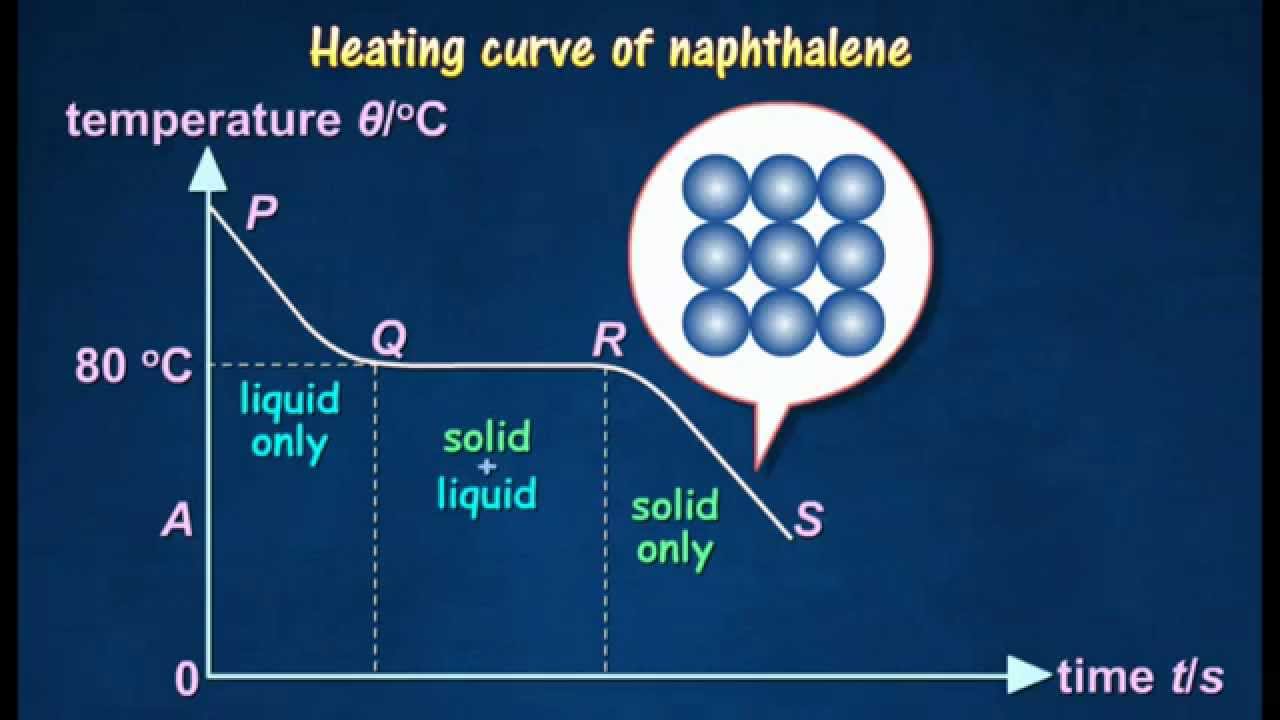
In this exercise you will measure cooling curves of either the napthalene-biphenyl system group 1 or the napthalene-durene 1 2 4 5-tetramethylbenzene system group 2. One way to check the purity of the separated liquid is to measure its boiling point. It volatilises and sublimes at room temperature above the melting point. Major point of concern with these pieces of Cold End Equipment is the potential problem of sulfuric acid condensation on surfaces cooler than the Acid Dew Point. In a covalent compound the covalent molecules are held together by weak forces of attraction. 800 C 1760 F AutoSelf-ignition temperature.

This causes 1 corrosion and 2 accumulation of soot and ash particles. 5 Introduction to Organic Chemistry go to 1. The point group symmetry of naphthalene is D 2h. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids. Open flames and high energy ignition sources.
 Source: sukachem.blogspot.com
Source: sukachem.blogspot.com
In many cases T b is unknown or difficult to measure. 1-2-Naphthylethanone beta -methyl naphthyl ketone. The point group symmetry of naphthalene is D 2h. One way to check the purity of the separated liquid is to measure its boiling point. The melting and boiling points of covalent compounds are low.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
STABILITY AND REACTIVITY STABILITY. 5 Introduction to Organic Chemistry go to 1. Beta -naphthyl methyl ketone. Material is stable under normal conditions. In this exercise you will measure cooling curves of either the napthalene-biphenyl system group 1 or the napthalene-durene 1 2 4 5-tetramethylbenzene system group 2.
5 Introduction to Organic Chemistry go to 1. Open flames and high energy ignition sources. Available heat in flue gas is less at low loads. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the. If you see two liquid layers add a little more solvent.
 Source: shaalaa.com
Source: shaalaa.com
Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the. It volatilises and sublimes at room temperature above the melting point. Chemistry is one of the fundamental sciences. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the. Previous year IIT JEE Fundamental Concepts Of Organic Chemistry Questions and answers are available.
 Source: researchgate.net
Source: researchgate.net
Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids. From these data you will generate the phase diagram for the system and determine Δ fus H and the melting point for the two pure substances in your system. The primary use for naphthalene is in the production of phthalic anhydride also of carbamate insecticides surface active agents and resins as a dye intermediate as a synthetic tanning agent as a moth repellent and in miscellaneous organic chemicals. For example pure water boils at 100C. Low-melting point solid Rigid polyurethane foam production Hexamethylene diisocyanate HDI Liquid Spray paints lacquers and car re-finishing Napthalene diisocyanate NDI Solid Elastomers and synthetic rubbers Methyl isocyanate MIC Liquid highly volatile Intermediate in the production of some pesticides Isophorone diisocyanate IPDI Liquid Manufacture of coating and adhesive polymers.
 Source: dailystudytips.com
Source: dailystudytips.com
The melting and boiling points of covalent compounds are low. Generally including barcode scanners cash drawers dedicated computers electronic funds transfer point of sale EFTPOS machines keyboards monitor s printers and terminals 6 years. Point of sale assets. Melting point C Boiling point C Ethanol C 2 H 5 OH-117. Open flames and high energy ignition sources.
 Source: youtube.com
Source: youtube.com
For example pure water boils at 100C. Available heat in flue gas is less at low loads. Melting point C Boiling point C Ethanol C 2 H 5 OH-117. Napthalene plant assets. Paraffin oil nondangrs flsh 244c above in tank wgn 200.
 Source: chemistry.analia-sanchez.net
Source: chemistry.analia-sanchez.net
Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Napthalene plant assets. This equation requires that the boiling point T b for the mixture of interest is known. Electric and steam pumps tar tanks strainers salt water coolers including supporting structures and distribution pipes to cooler off takes including valves 40 years. Some air may have to bypass the air heater to avoid chilling the metal below the dew point.
 Source: researchgate.net
Source: researchgate.net
While all the effort has been made to make this. Some air may have to bypass the air heater to avoid chilling the metal below the dew point. Open flames and high energy ignition sources. In many cases T b is unknown or difficult to measure. 795 - 810 C 1751 - 1778 F Solubilities.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title napthalene melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.