Naphthalene boiling point
Home » datasheet » Naphthalene boiling pointNaphthalene boiling point
Naphthalene Boiling Point. Compound Hydrocarbon Class Formula Boiling. 218 C Alfa Aesar A13188 33347. Naphthalene is an organic compound with formula C 10 H 8. 218 C LabNetwork old LN00194168.
2040 595 179 40 K f. This affects the freezing and boiling temperatures of the solution by lowering the freezing point and increases the boiling point in relation to the pure solventThe unknown substance properties depend on the number of solute particles and not the properties of the solute particles these properties are called colligative properties. The Occupational Safety and Health Administration OSHA has defined a flammable liquid as any liquid having a flash point of not more than 93 C or 1994 F 29CFR 1910106a19. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. In this experiment you will be. 29 CFR 1910106a19i-v and Table B61 of 29 CFR 19101200 Appendix B.
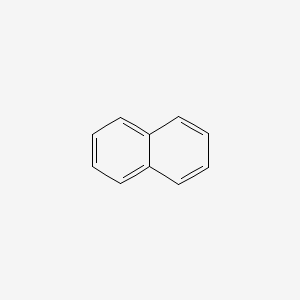
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases.
All boiling points below are normalatmospheric boiling points. This affects the freezing and boiling temperatures of the solution by lowering the freezing point and increases the boiling point in relation to the pure solventThe unknown substance properties depend on the number of solute particles and not the properties of the solute particles these properties are called colligative properties. Be placed in boiling water for 30 minutes but make sure that none of the water is able to enter the tubes. Naphthalene is an organic compound with formula C 10 H 8. 423-425 F 760 mmHg 2172222-2183333 C 760 mmHg Wikidata Q179724. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure.
 Source: researchgate.net
Source: researchgate.net
If you wish to use plants other than cauliflower you need to prepare two different media which contain plant hormones necessary to stimulate development of differentiated tissues. We would like to show you a description here but the site wont allow us. 218 C LabNetwork old LN00194168. Table 2 Boiling and melting points of representative diesel fuel hydrocarbons. Be placed in boiling water for 30 minutes but make sure that none of the water is able to enter the tubes.
 Source: researchgate.net
Source: researchgate.net
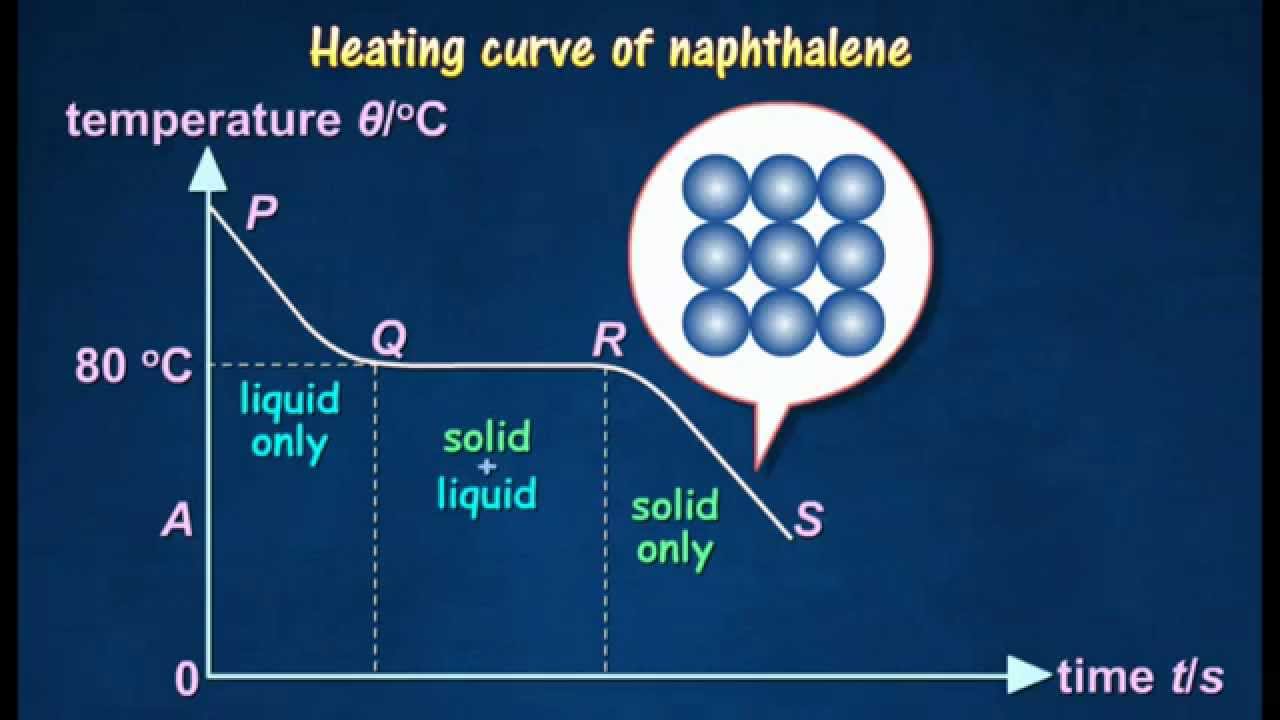
218 C FooDB FDB000954. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. All boiling points below are normalatmospheric boiling points. An impure compound typically melts at a lower temperature and over a broader range of temperatures.
 Source: fishersci.co.uk
Source: fishersci.co.uk
The relationship between pressure change and temperature change during evaporation in general. 423-425 F 760 mmHg 2172222-2183333 C 760 mmHg Wikidata Q179724. Calculate the approximate initial boiling point in o C of a solution of 285 g of magnesium chloride in 20 kg of water. The category of a flammable is determined by its flash point and boiling point. 218 C OU Chemical Safety Data No longer updated More details.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Assume complete dissociation of the salt a 1031 o C b 1016 o C c 1023 o C d 1008 o C e 1048 o C 12. Assume complete dissociation of the salt a 1031 o C b 1016 o C c 1023 o C d 1008 o C e 1048 o C 12. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor.
Source: chemspider.com
An open tank would typically be used for cleaning at less than the boiling point cold cleaning. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. The benzoic acid will also be recrystallized using boiling water and will then be weighed and measured for its melting point. Dominant strongest type of IMF for the pure. Boiling Point Elevation and Freezing Point Depression.

Molality m is the concentration of the solute expressed by. The first one should contain a cytokinin. The category of a flammable is determined by its flash point and boiling point. Compound Hydrocarbon Class Formula Boiling. 1560 626 306 Camphor.
Source: pubchem.ncbi.nlm.nih.gov
1843 369 596 587 K b K f. 424 F 760 mmHg 2177778 C 760. 1560 626 306 Camphor. Therefore taking a melting point can aid in identifying an organic compound and assessing its purity. As an aromatic hydrocarbon naphthalenes structure consists of a fused pair of benzene rings.
 Source: clutchprep.com
Source: clutchprep.com
Dominant strongest type of IMF for the pure. Boiling Point Elevation and Freezing Point Depression. Compound like boiling point density and refractive index. The temperature of the column does not have to be above the boiling point because every compound has a non-zero vapor pressure at any given temperature even solids. 3 CF 3 c.

An impure compound typically melts at a lower temperature and over a broader range of temperatures. Naphthalene is an organic compound with formula C 10 H 8. 1179 307 166 390 K b K f. Some fuels and their boiling points at atmospheric pressure. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher.
 Source: youtube.com
Source: youtube.com
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. 2989 44 39 Acetic acid. 424 F 2177778 C NIOSH QJ0525000. All boiling points below are normalatmospheric boiling points.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title naphthalene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.