Naoh boiling point
Home » datasheet » Naoh boiling pointNaoh boiling point
Naoh Boiling Point. Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a. Stir the water continuously while adding the salt mixture. Add 30 g sugar and stir to dissolve. The pH is adjusted to 57 using 01 M HCl or NaOH.
 Boiling Temperature At Atmospheric Pressure Of Aqueous Solutions Of Download Scientific Diagram From researchgate.net
Boiling Temperature At Atmospheric Pressure Of Aqueous Solutions Of Download Scientific Diagram From researchgate.net
The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Dissolve the MS mixture in about 800 ml of distilled water. Distillation is recommended in the case of liquids see Appendix 3. A gram equivalent weight or equivalent is a measure of the reactive capacity of a given molecule. D the pH change occurs outside the range of any indicator. It serves the dual purpose of determining the bp as well as purification of the liquid for subsequent tests.
The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F.
Total for Question 13 1 mark 14 Which of the following reagents could be used to. The process for distillation involves boiling the water allowing the steam to condense in a tube and collecting the condensation in a container. Solid NaOH has a melting point and boiling point of approximately 604F and 2534F. When dissolving solid NaOH in water or a strong acid. Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F.
3 NaOH NaNO 3 H 2O B HNO 3 H 2O H 3O. The solubility of the unknown in. 3 NaOH NaNO 3 H 2O B HNO 3 H 2O H 3O. Distillation is recommended in the case of liquids see Appendix 3. The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide.
Source:
2 Na 2 O 2 2 Na 2 O O 2 Preparation. It serves the dual purpose of determining the bp as well as purification of the liquid for subsequent tests. Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14. Analysis for elements present. Normality is the only.
 Source: magnusequipment.com
Source: magnusequipment.com
D the pH change occurs outside the range of any indicator. 3 NaOH NaNO 3 H 2O B HNO 3 H 2O H 3O. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. There may be many substances dissolved in the water and some of them may vaporize along with the water but salts and other solid solutes are left behind. Total for Question 13 1 mark 14 Which of the following reagents could be used to.
 Source: researchgate.net
Source: researchgate.net
Sophisticated distillation techniques can eliminate even the volatile solutes and if you. 1390 deg C 760 mmHg FreezingMelting Point318 deg C Decomposition TemperatureNot available. 3 NaOH NaNO 3 H 2O B HNO 3 H 2O H 3O. Normality is the only. A gram equivalent weight or equivalent is a measure of the reactive capacity of a given molecule.
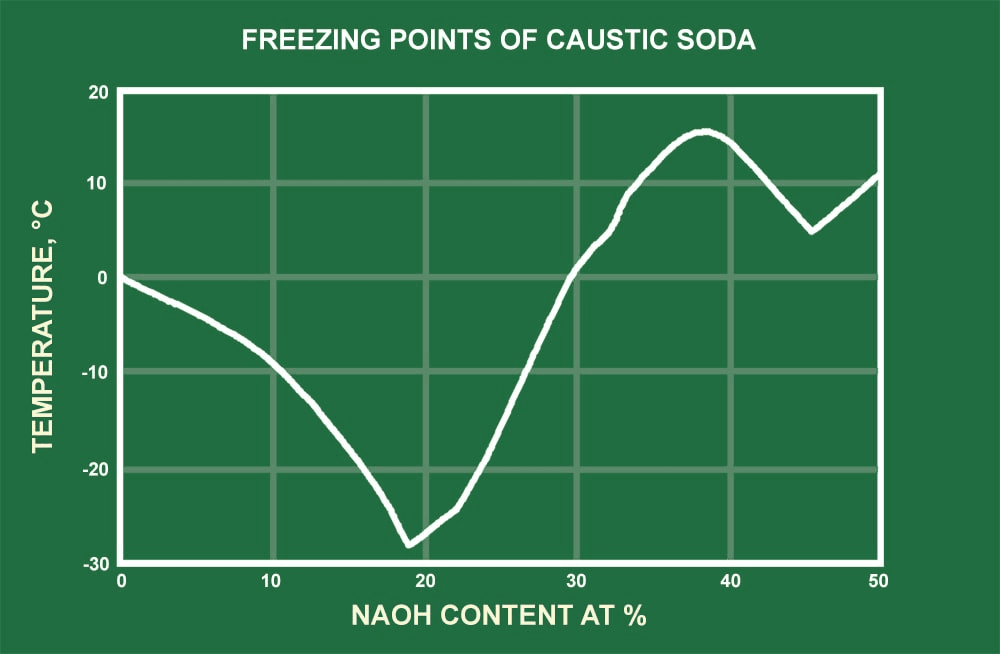 Source: protank.com
Source: protank.com
Add 30 g sugar and stir to dissolve. The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H—O- hydrogen bonding in alcohols light blue columns. The solubility of the unknown in. 2 Na 2 O 2 2 Na 2 O O 2 Preparation. Dissolve the MS mixture in about 800 ml of distilled water.
 Source: researchgate.net
Source: researchgate.net
10 g NaOH 40 g NaOH 1 mol NaOH 025 mol NaOH 500 g water x 1 kg 1000 g 050 kg water molality 025 mol 050 kg molality 005 M kg molality 050 m Normality N Normality is equal to the gram equivalent weight of a solute per liter of solution. Boiling Point and Water Solubility. Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14. Analysis for elements present. It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers.
 Source: researchgate.net
Source: researchgate.net
Determine the boiling point or melting point. With further heating above the 657 C boiling point the compound decomposes to Na 2 O releasing O 2. At C10 level the elements present will be told to you but read up the method. The amount of solute that can be dissolved in solvent is called its solubilityFor example in a saline solution salt is the solute dissolved in water as the solvent. 10 g NaOH 40 g NaOH 1 mol NaOH 025 mol NaOH 500 g water x 1 kg 1000 g 050 kg water molality 025 mol 050 kg molality 005 M kg molality 050 m Normality N Normality is equal to the gram equivalent weight of a solute per liter of solution.
 Source: protank.com
Source: protank.com
The amount of solute that can be dissolved in solvent is called its solubilityFor example in a saline solution salt is the solute dissolved in water as the solvent. Procedure Preparation and sterilization of growing medium when not provided pre-poured These steps will make 1 L of growth medium which is enough to prepare about 65 growing tubes. Solid NaOH has a melting point and boiling point of approximately 604F and 2534F. It serves the dual purpose of determining the bp as well as purification of the liquid for subsequent tests. It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers.
 Source: researchgate.net
Source: researchgate.net
Stir the water continuously while adding the salt mixture. When dissolving solid NaOH in water or a strong acid. The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H—O- hydrogen bonding in alcohols light blue columns. The end-point because A the pH change is too gradual close to the equivalence point. Silver nitrate is an inorganic compound with chemical formula AgNO 3This salt is a versatile precursor to many other silver compounds such as those used in photographyIt is far less sensitive to light than the halidesIt was once called lunar caustic because silver was called luna by the ancient alchemists who associated silver with the moon.
 Source: researchgate.net
Source: researchgate.net
Procedure Preparation and sterilization of growing medium when not provided pre-poured These steps will make 1 L of growth medium which is enough to prepare about 65 growing tubes. It serves the dual purpose of determining the bp as well as purification of the liquid for subsequent tests. The amount of solute that can be dissolved in solvent is called its solubilityFor example in a saline solution salt is the solute dissolved in water as the solvent. A gram equivalent weight or equivalent is a measure of the reactive capacity of a given molecule. It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title naoh boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.