Nabh4 melting point
Home » datasheet » Nabh4 melting pointNabh4 melting point
Nabh4 Melting Point. Bond-line Lewis and Condensed Structures with Practice Problems. Sodium borohydride is a weaker reducing agent than lithium aluminum hydride because th e B-H bond is less polar than the Al -H bond. The melting point of gold. CID 28123 Borohydride CID 5360545 Sodium Dates.
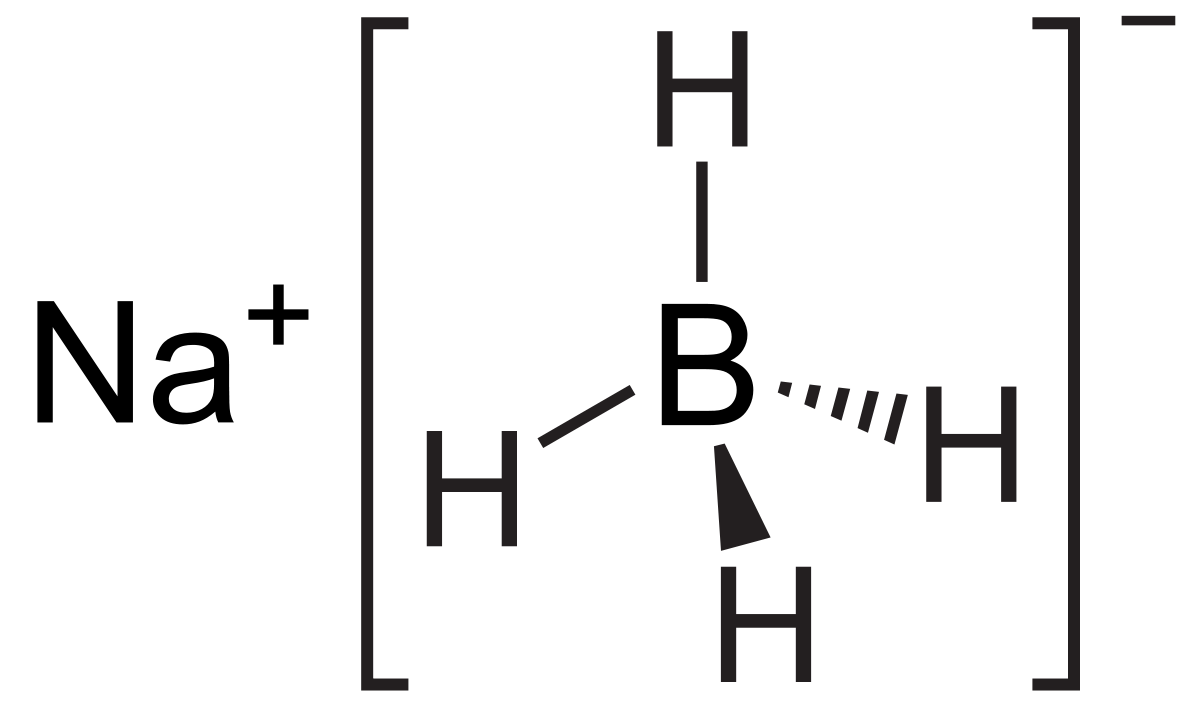 Sodium Borohydride Wikipedia From en.wikipedia.org
Sodium Borohydride Wikipedia From en.wikipedia.org
The purity could be. This table contains information on each unique technology nominated for the Green Chemistry Challenge from 1996 through 2019. What is the melting point of the compound depicted in the graphic. Infrared spectra were recorded on a PerkinElmer Fourier transform infrared FT-IR RXI spectrophotometer. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. Sodium borohydride NaBH4 and lithium aluminum hydride LAH LiAlH.
Ion coaters are an easy and direct method for generating uniform gold nanoparticles with a narrow size distribution by combining an ion coater on.
Umehara M Hanada A Yoshida S Akiyama K Arite T et al. Upon topical application benzoyl peroxide decomposes to release oxygen which is lethal to the bacteria Proprionibacterium acnes. What is the melting point of the compound depicted in the graphic. Prior to the loss of product in step 2 the overall yield of the synthesis was 174. The purity could be. Bond-Line or Skeletal Structures.
Heating mgso4 7h2o. Version 12 just got released with a host of corrections and a new page index. Sodium borohydride NaBH4 and lithium aluminum hydride LAH LiAlH. Use the question 12 graphic. Prior to the loss of product in step 2 the overall yield of the synthesis was 174.
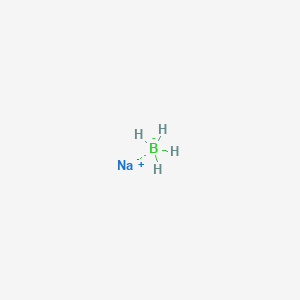
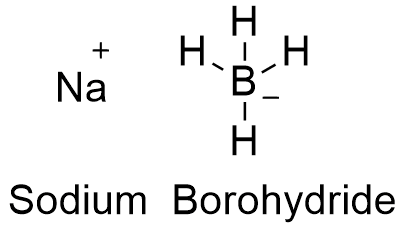 Source: softschools.com
Source: softschools.com
Resonance Structures in. Due to its irritant effect benzoyl peroxide increases turnover rate of epithelial cells thereby peeling the skin and promoting the resolution of. That is the first step in the reaction is coordination of a lone pair from the carbonyl oxygen a nucleophile to the aluminum electrophile. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. 483 to 486 K Hazards NFPA 704 fire diamond 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Having just talked about the oxidation ladder it makes sense to start going into. Volume πr2h. Having just talked about the oxidation ladder it makes sense to start going into. Curved Arrows with Practice Problems. H 2 x 10 6 m 2 x 10 4 cm S S S Calculation.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Having just talked about the oxidation ladder it makes sense to start going into. The purity could be. These reducing agents contain a metal hydrogen bond that serve as a source of the hydride ion a good nucleophile. This low yield is mostly contributed to the high concentrations of unreacted isobutylbenzene within the first step. BRILLIANT PUBLIC SCHOOL SITAMARHI Affiliated up to 2 level to CBSE New Delhi Class-XI IIT-JEE Advanced Chemistry Study Package Session.
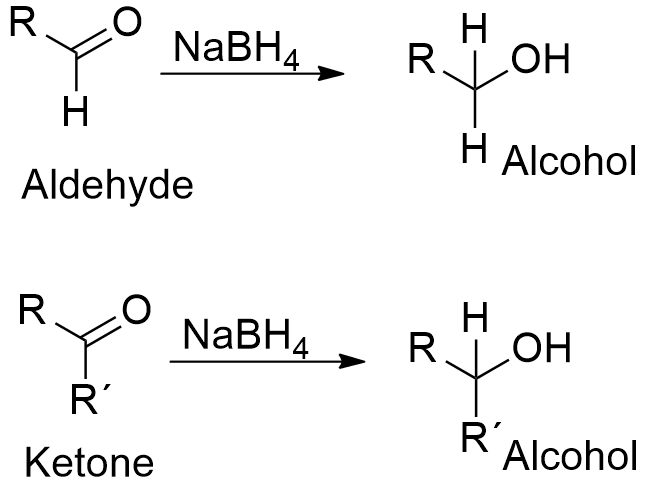 Source: softschools.com
Source: softschools.com
Sodium borohydride is a weaker reducing agent than lithium aluminum hydride because th e B-H bond is less polar than the Al -H bond. Related compounds Related compounds. Sodium borohydride NaBH4 and lithium aluminum hydride LAH LiAlH. Cook CE Whichard LP Turner B Wall ME Egley GH 1966 Germination of 4. 78 gmol melting point of 290C and λ max of 540nm.
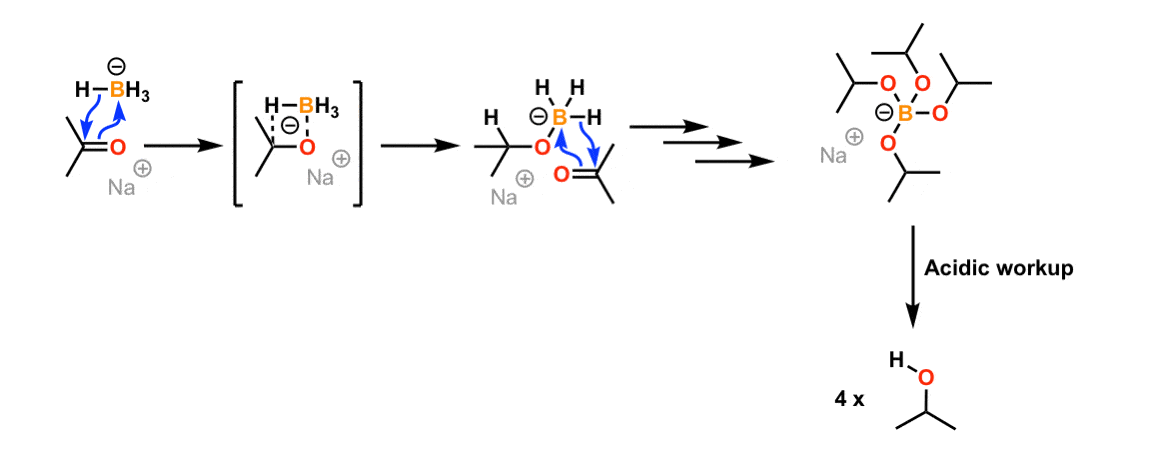 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
33 x 10S4 cm22 x 10S4 cm. Ion coaters are an easy and direct method for generating uniform gold nanoparticles with a narrow size distribution by combining an ion coater on. This table contains information on each unique technology nominated for the Green Chemistry Challenge from 1996 through 2019. Due to its irritant effect benzoyl peroxide increases turnover rate of epithelial cells thereby peeling the skin and promoting the resolution of. 2008 Witchweed Striga lutea Lour.
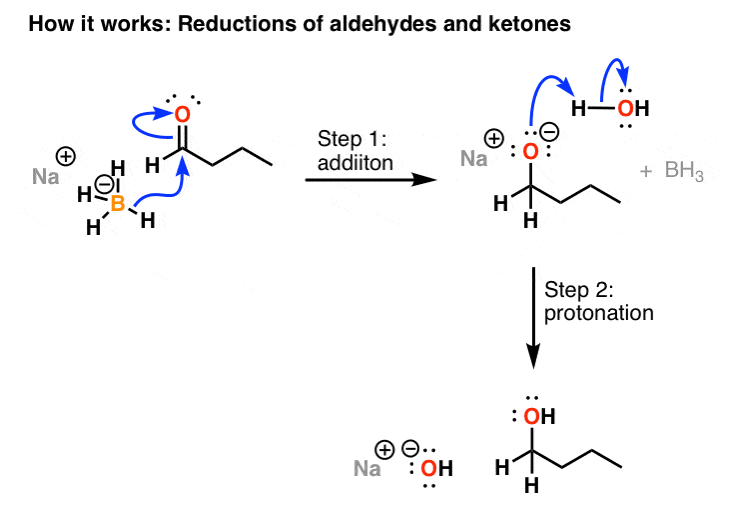 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Sodium borohydride NaBH4 and lithium aluminum hydride LAH LiAlH. These reducing agents contain a metal hydrogen bond that serve as a source of the hydride ion a good nucleophile. Related compounds Related compounds. Sodium borohydride NaBH4 and lithium aluminum hydride LAH LiAlH. Version 12 just got released with a host of corrections and a new page index.
 Source: chemyq.com
Source: chemyq.com
Boiling Point and Melting Point in Organic Chemistry. Heating mgso4 7h2o. Sodium borohydride is a white to grayish crystalline powder. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. Having just talked about the oxidation ladder it makes sense to start going into.
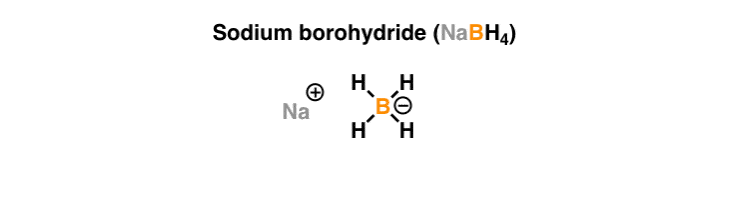 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Functional Groups in Organic Chemistry with Practice Problems. Heating mgso4 7h2o. Due to its irritant effect benzoyl peroxide increases turnover rate of epithelial cells thereby peeling the skin and promoting the resolution of. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. Use the question 12 graphic.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium borohydride is a white to grayish crystalline powder. Benzoyl Peroxide is a peroxide with antibacterial irritant keratolytic comedolytic and anti-inflammatory activity. Bond-Line or Skeletal Structures. Calcium oxide CaO Bendosen Laboratory Chemicals and ammonium dihydrogen phosphate NH4H2HPO4 Friendmann Schmidt Chemical were used to produce hydroxyapatite powder. That is the first step in the reaction is coordination of a lone pair from the carbonyl oxygen a nucleophile to the aluminum electrophile.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nabh4 melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.