N butanol boiling point
Home » datasheet » N butanol boiling pointN butanol boiling point
N Butanol Boiling Point. For example diethyl ether CH 3 CH 2 OCH 2 CH 3 has a boiling point of 346 o C whereas n-butanol CH 3 CH 2 CH 2 CH 2 OH a four carbon alcohol has a boiling piont of 1177 o C. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. COMPOSITION Components CAS Weight percent n-Butanol 71-36-3 9985 - 100 Isobutanol or other Alcohols 78-83-1 02 max Water KARL FISCHER METHOD 01 max Toxicological Data on Ingredients. Temperature K A B C Reference Comment.
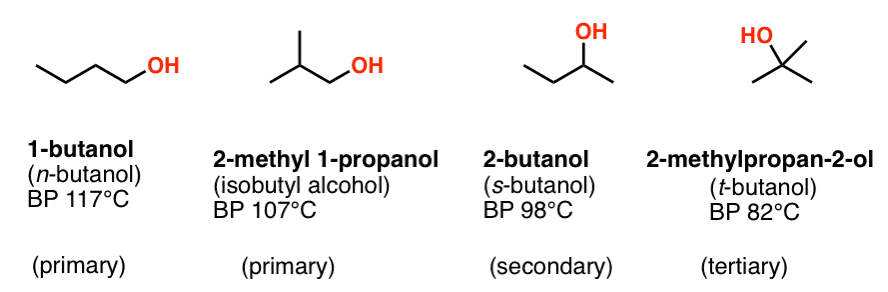 Alcohols 1 Nomenclature And Properties Master Organic Chemistry From masterorganicchemistry.com
Alcohols 1 Nomenclature And Properties Master Organic Chemistry From masterorganicchemistry.com
Any glovechemical combination does not meet either set of conditions required for a GREEN or RED rating. Temperature K A B C Reference Comment. Check for and remove any. However DEHP is listed by the IARC as a human carcinogen. It is a clear colourless transparent liquid that has a typical sharp musty odour that is comparable with the. DEHP offers good gelling satisfactory electrical properties and helps to produce highly elastic compounds with reasonable cold strength.
Ideally the selected solvents should be safe to use non-flammable and non-toxic in both liquid and vapor states.
It can also be used as a solvent in coatings. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. The boiling point of n-butanol is 117 o C. ILO International Chemical Safety Cards ICSC-050 C. It is a versatile solvent featuring excellent reactivity as a chemical intermediate in the. 71 -36 -3 Molecular Weight.
 Source: en.wikipedia.org
Source: en.wikipedia.org
However DEHP is listed by the IARC as a human carcinogen. 1560 626 306 Camphor. N-propanol also known as 1-propanol n-propanol alcohol propan-1-ol propyl alcohol is a primary alcohol in which the OH entity is bonded to a primary carbon atom. DEHP has been. 23 K to 353.
 Source: iea-amf.org
Source: iea-amf.org
Direction of Heat. CH3CH22CH2OH COMPANY IDENTIFICATION Supplier. COMPOSITION Components CAS Weight percent n-Butanol 71-36-3 9985 - 100 Isobutanol or other Alcohols 78-83-1 02 max Water KARL FISCHER METHOD 01 max Toxicological Data on Ingredients. N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n. Boiling Point Volatility Temp.
 Source: researchgate.net
Source: researchgate.net
7412 Chemical Formula. Toluene Xylene Naphatha Solvent in water Water in solvent Dilution rate Blushing resistance a 25ºC UR Solubility at 20ºC weight Distillation range at 760 mmHg ºC 2 Freezing point ºC Absolute viscosity at 20ºC. DEHP has been. 23 K to 353. Supplementary information for Comprehensive.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
3 always be considered. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. 801 265 55 512 K b K f. Supplementary information for Comprehensive. Waste disposal either by evaporation into the air or liquid disposal into wastewater should.
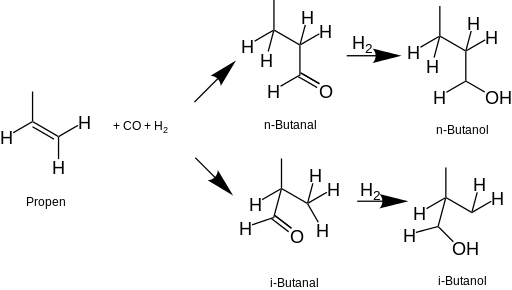 Source: en.wikipedia.org
Source: en.wikipedia.org
Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. Energy Forms. It can also be used as a solvent in coatings. Mass and Surface Area Affect the Strength of London Dispersion Forces. Toluene Xylene Naphatha Solvent in water Water in solvent Dilution rate Blushing resistance a 25ºC UR Solubility at 20ºC weight Distillation range at 760 mmHg ºC 2 Freezing point ºC Absolute viscosity at 20ºC.
 Source: iea-amf.org
Source: iea-amf.org
This reaction occurred through the S. Hessel and Geiseler 1965. The selected solvents should have a relatively low boiling point to allow low temperature evaporation and leave no residue. Check for and remove any. Structure of DEHP.
 Source: iea-amf.org
Source: iea-amf.org
It is a clear colourless transparent liquid that has a typical sharp musty odour that is comparable with the. 462 234 1115. National Toxicology Program Chemical Repository Database. However DEHP is listed by the IARC as a human carcinogen. 23 K to 353.
Page 1 of 7 MSDS N-Butanol Material Safety Data Sheet MSDS- N-BUTANOL 1. Product Identification Synonyms. 250 - 257C at 05 kPa. Research Triangle Park North Carolina. CH3CH22CH2OH COMPANY IDENTIFICATION Supplier.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Fig 1 benzoic acid solution Erlenmeyer flask hot plate Fig 1- Dissolving benzoic acid Remove the flask from the hot plate and. Ideally the selected solvents should be safe to use non-flammable and non-toxic in both liquid and vapor states. Thus ethers containing up to 3 carbon atoms are soluble in water due to the. 2-Ethyl Hexanol is a branched chain alcohol with a high boiling point and slow evaporation rate. As a result the boiling point of neopentane 95C is more than 25C lower than the boiling point of n-pentane 361C.
Source: chemspider.com
2-Ethyl Hexanol is a branched chain alcohol with a high boiling point and slow evaporation rate. But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. Product Identification Synonyms. Energy Forms. Check for and remove any.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title n butanol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.