Methylene unit and boiling point
Home » datasheet » Methylene unit and boiling pointMethylene unit and boiling point
Methylene Unit And Boiling Point. Computed by PubChem release 20210507 PubChem. Dibromomethane appears as a colorless liquid with a pleasant odor. Used as a solvent and as a. 12 point ArialTimes New Roman.
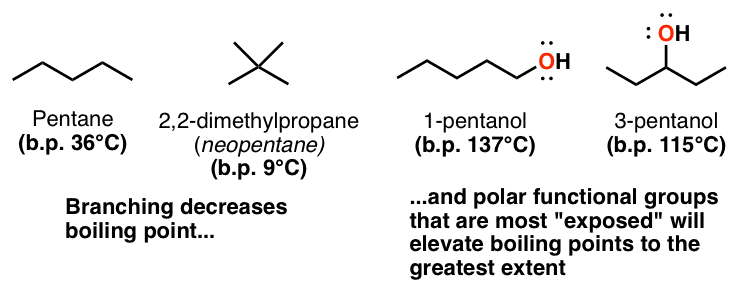 3 Trends That Affect Boiling Points Master Organic Chemistry From masterorganicchemistry.com
3 Trends That Affect Boiling Points Master Organic Chemistry From masterorganicchemistry.com
Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2. In seven subjects exposed to 710 mgcu m for 2 hr the corresponding value was 815 ug. 12 point ArialTimes New Roman. To do this the substance to be recrystallized is placed in an. May be toxic by ingestion. LD50 Rat 5045 mgkg.
Dibromomethane appears as a colorless liquid with a pleasant odor.
Home Christmas Lights Bubble. The shapes of stearic and oleic acids are displayed in the models below. Isobutane which has a dipole moment near zero has a low boiling point of 117 C. Double and single spacing. Usually recrystallization is carried out by first dissolving the solid in a boiling hot solvent. You may examine models of these compounds by clicking on the desired model picture.
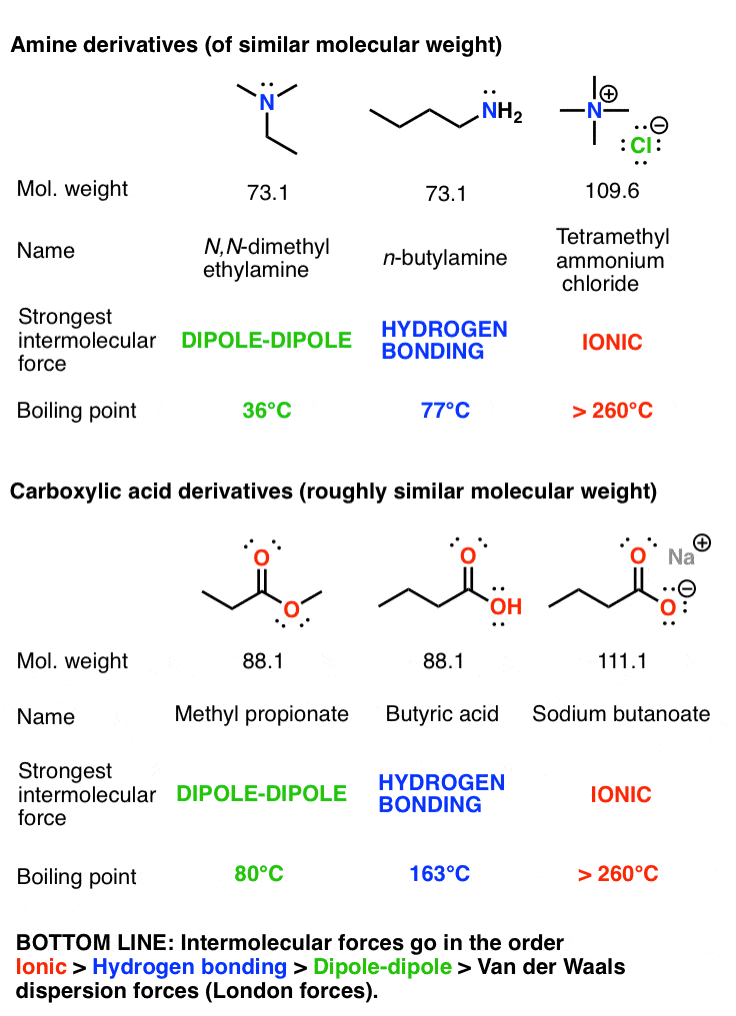 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The molecular weights are H 2 2 CO 28 HF 20 and Ne 20. What advantages do you get from our Achiever Papers services. Natural sources of dichloromethane include oceanic sources macroalgae wetlands and. In four human subjects exposed to methylene chloride 350 mgcu m for 2 hr an average of 226 microg methylene chloride was excreted in the urine within 24 hr after the exposure. A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact thus the greater van der Waals forces between adjacent molecules.
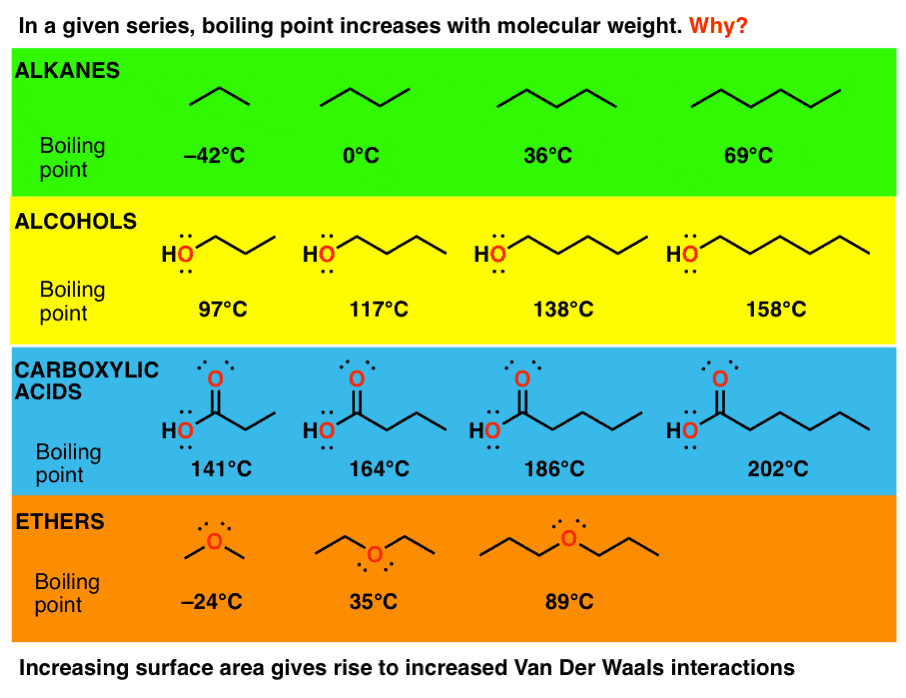 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The physical properties of these two molecules reflect their dipole moments. In four human subjects exposed to methylene chloride 350 mgcu m for 2 hr an average of 226 microg methylene chloride was excreted in the urine within 24 hr after the exposure. The trans-double bond isomer of oleic acid known as elaidic acid has a linear shape and a melting point of 45 ºC 32 ºC higher than its cis isomer. Which of the compounds will react faster in S N 1 reaction with the OH ion. The attractive forces are stronger for ionic substances than for molecular ones The intermolecular forces of the remaining substances depend on molecular weight polarity and hydrogen bonding.
Usually recrystallization is carried out by first dissolving the solid in a boiling hot solvent. Below enter the boiling point. Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2. Example 1 Distillation of Methylene Di Chloride with water Explanation In a process the separation of Methylene Di Chloride with water using a liquid-liquid separation technique can be called as a unit operation Explanation In the above process only separation is taking place which will be done base on density difference. Ideally the solute is very soluble in the solvent at its boiling point but virtually insoluble at 0C.
 Source: researchgate.net
Source: researchgate.net
LD50 Rabbit 12800 mgkg. For example compare isobutane 2-methylpropane and n-butane butane which boil at 12 and 0 C and 22-dimethylbutane and 23-dimethylbutane which boil at 50 and 58 C respectively. Below enter pressure for a known boiling point. Initially the calculator is set to evaluate results for water and substances of similar heat of evaporation DMF aniline toluene CALIBRATION. The shapes of stearic and oleic acids are displayed in the models below.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2. For example compare isobutane 2-methylpropane and n-butane butane which boil at 12 and 0 C and 22-dimethylbutane and 23-dimethylbutane which boil at 50 and 58 C respectively. 43 hPa 20 C Dimensions. Isobutane which has a dipole moment near zero has a low boiling point of 117 C. Acetone however has a large dipole moment of 291 D and a boiling point of 56-57 C.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The boiling point of H 2 should be the lowest. You may examine models of these compounds by clicking on the desired model picture. Isobutane which has a dipole moment near zero has a low boiling point of 117 C. Methylene chloride is removed from the body mainly in expired air and urine. Ideally the solute is very soluble in the solvent at its boiling point but virtually insoluble at 0C.
 Source: byjus.com
Source: byjus.com
Hence high recovery is only an option when operating a second-pass RO unit or when the feed water is pure. From a process point of view higher recovery results in higher salt concentration in the feedreject channel that in turn results in sparingly soluble salts exceeding their solubility limits faster. Two polyunsaturated fatty acids linoleic and linolenic are. The physical properties of these two molecules reflect their dipole moments. Usually recrystallization is carried out by first dissolving the solid in a boiling hot solvent.
 Source: sciencedirect.com
Source: sciencedirect.com
We would like to show you a description here but the site wont allow us. From a process point of view higher recovery results in higher salt concentration in the feedreject channel that in turn results in sparingly soluble salts exceeding their solubility limits faster. Below enter the boiling point. Acetone however has a large dipole moment of 291 D and a boiling point of 56-57 C. Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2.
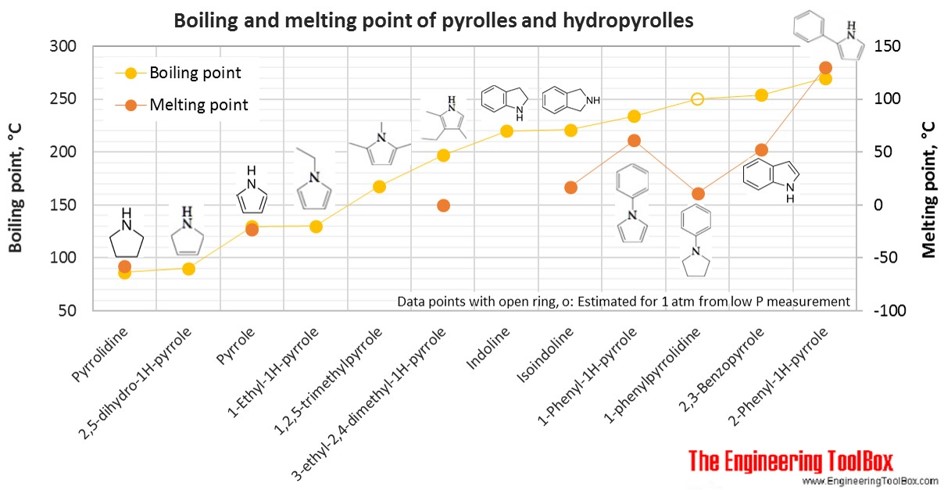 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
May be toxic by ingestion. In seven subjects exposed to 710 mgcu m for 2 hr the corresponding value was 815 ug. To do this the substance to be recrystallized is placed in an. Used as a solvent and as a. Safety Information according to GHS.
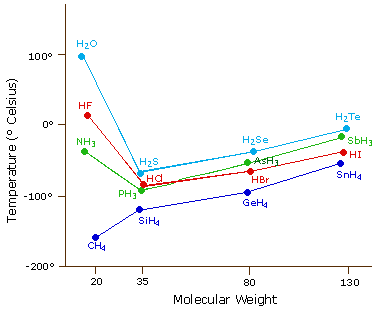 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
Safety Information according to GHS. The trans-double bond isomer of oleic acid known as elaidic acid has a linear shape and a melting point of 45 ºC 32 ºC higher than its cis isomer. The result will be displayed in the same units. Out of o-and p-dibromobenzene which one has higher melting point and why. 9712 orders delivered before the deadline.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title methylene unit and boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.