Methylene chloride melting point
Home » datasheet » Methylene chloride melting pointMethylene chloride melting point
Methylene Chloride Melting Point. NR Not. 1 Production of R11 or CFC-11 was halted by the clean air act on January 1 1996 2 Production of R12 or CFC-12 Dichlorodifluoromethane was halted by the clean air act on January 1 1996 3 R22 or HCFC-22 is a single component HCFC refrigerant with low ozone depletion potential. 137 ºC and B is benzoic acid mp. The American Conference of Governmental Industrial Hygienists recommends.

If so they can be combined. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid. Melting Point C Physical Form. _____ Page 2 9 _____ Oxalyl chloride 20M solution in dichloromethane Revision Date 28-Nov. Calibration stock solution 025 mgmL Check purity of each PAH reference standard by GCFID HPLCfluorescence andor melting point. Load dried caffeine reference powder into a capillary tube and tap the capillary tube on a hard surface until the sample packs into the bottom.
It has long been used in a variety of air-conditioning and refrigeration applications in a variety of markets.
If you are not sure which layer is the organic or the aqueous layer perform the water drop test. Accordingly the concentration of methylene chloride in the air inside a foam plant must be kept low. If not keep them separate. Chloroform and methylene chloride are denser than water while most other organic solvents are not as dense as water. Methylene chloride is a Lewis acid that can hydrogen bond to electron donors. If so they can be combined.
 Source: softschools.com
Source: softschools.com
If not keep them separate. Then extracted from the water with dichloromethane methylene chloride which is an organic solvent that is insoluble in water. Check the chemical compatibility of Polyvinyl chloride PVC with various chemicals solvents alcohols and other products. Measuring Melting point 1. Melting Point-1421 F NTP 1992 Vapor Pressure.
 Source: en.wikipedia.org
Source: en.wikipedia.org
_____ Page 2 9 _____ Oxalyl chloride 20M solution in dichloromethane Revision Date 28-Nov. The melting point of PVC is low around 100C 212F Maximum operating temperature is around 60C 140F. Methylene chloride 75-09-2 75 Ethanedioyl dichloride 79-37-8 25 4. If so they can be combined. The above products are stable if stored under recommended conditions.

Accordingly the concentration of methylene chloride in the air inside a foam plant must be kept low. Interim AEGLs for Methylene chloride 75-09-2 Exposure Period AEGL-1 AEGL-2 AEGL-3. Methylene chloride is highly volatile boiling point 398C and inert in polyurethane-forming mixtures. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available. Boiling Point C Feature.
 Source: acs.org
Source: acs.org
If not keep them separate. It is classified as a hard acid and is included in the ECW model. Melting Point-1421 F NTP 1992 Vapor Pressure. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. It has a role as an EC 1434 monoamine oxidase inhibitor an acid-base indicator a fluorochrome an antidepressant a cardioprotective agent an EC 3118.
Thus the melting points of triglycerides reflect their composition as shown by the following examples. Melting point or other methods eg thin layer chromatography to determine whether the purity of the second crop is equal to that of the first. Load dried caffeine reference powder into a capillary tube and tap the capillary tube on a hard surface until the sample packs into the bottom. Calibration stock solution 025 mgmL Check purity of each PAH reference standard by GCFID HPLCfluorescence andor melting point. Interim AEGLs for Methylene chloride 75-09-2 Exposure Period AEGL-1 AEGL-2 AEGL-3.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations. 4 Methylene Chloride-d 2 Pyridine-d 5 1122 Tetrachloroethane-d 2 Toluene-d 8 Trifluoroacetic Acid-d 222-Trifluoroethanol-d 3 Store at room temperature away from light and moisture. But we want to separate the caffeine from the tannins by. Load dried caffeine reference powder into a capillary tube and tap the capillary tube on a hard surface until the sample packs into the bottom. Thus the melting points of triglycerides reflect their composition as shown by the following examples.
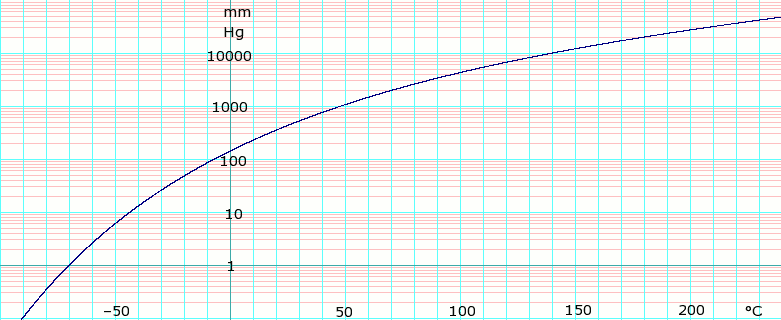 Source: en.wikipedia.org
Source: en.wikipedia.org
It has long been used in a variety of air-conditioning and refrigeration applications in a variety of markets. However methylene chloride is a suspected carcinogen and has other deleterious effects on workers exposed to it. National Toxicology Program Chemical Repository Database. Water amides NN-dimethylformamide alcohols methanol ethanol ketones acetone methyl ethyl ketone esters ethyl acetate chlorocarbons methylene. However the tannins are slightly soluble in the dichloromethane.

A small amount of compound B in a sample of compound A lowers and broadens its melting point. Chloroform and methylene chloride are denser than water while most other organic solvents are not as dense as water. It has a role as an EC 1434 monoamine oxidase inhibitor an acid-base indicator a fluorochrome an antidepressant a cardioprotective agent an EC 3118. Then extracted from the water with dichloromethane methylene chloride which is an organic solvent that is insoluble in water. Methylene chloride is a Lewis acid that can hydrogen bond to electron donors.
 Source: chemicals.ie
Source: chemicals.ie
Thus the melting points of triglycerides reflect their composition as shown by the following examples. The above products are stable if stored under recommended conditions. If so they can be combined. Methylene chloride is a Lewis acid that can hydrogen bond to electron donors. The lowest mixture melting point e is called the eutectic point.

It has a role as an EC 1434 monoamine oxidase inhibitor an acid-base indicator a fluorochrome an antidepressant a cardioprotective agent an EC 3118. Melting Point-1421 F NTP 1992 Vapor Pressure. Load dried caffeine reference powder into a capillary tube and tap the capillary tube on a hard surface until the sample packs into the bottom. Methylene chloride 2000 mgkg Rat 2000 mgkg Rat 53 mgL Rat 6 h 76000 mgm3 Rat 4 h Toxicologically Synergistic Products No information available Delayed and immediate effects as well as chronic effects from short and long-term exposure Irritation. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title methylene chloride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.