Methylene chloride boiling point
Home » datasheet » Methylene chloride boiling pointMethylene chloride boiling point
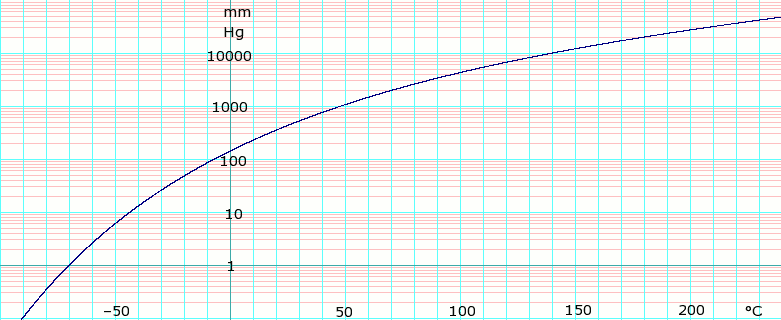
Methylene Chloride Boiling Point. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. Water boils at 18C under 15 millimeters. Natural sources of dichloromethane include oceanic sources macroalgae wetlands and. CRC Handbook of Chemistry and Physics.
Journal Library Iisc Ernet In From
_____ Page 2 9 _____ Oxalyl chloride 20M solution in dichloromethane Revision Date 28-Nov. Therefore the organic layer could be above or below the aqueous layer depending on the organic solvent used. If you are not sure which layer is the organic or the aqueous layer perform the water drop test. Since caffeine is more soluble in dichloromethane 140 mgml than it is in water 22 mgml it readily dissolves in the dichloromethane. The ICSC project is a common undertaking between the World Health Organization WHO and. You can now roughly evaluate its boiling point.
The main target users are workers and those responsible for occupational safety and health.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 106 C 223 F. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Natural sources of dichloromethane include oceanic sources macroalgae wetlands and. Chloroform and methylene chloride are denser than water while most other organic solvents are not as dense as water. Acetic acid anhydride CH 3 COO 2 O.
 Source: en.wikipedia.org
Source: en.wikipedia.org
You can now roughly evaluate its boiling point. Since caffeine is more soluble in dichloromethane 140 mgml than it is in water 22 mgml it readily dissolves in the dichloromethane. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Natural sources of dichloromethane include oceanic sources macroalgae wetlands and. If you are not sure which layer is the organic or the aqueous layer perform the water drop test.
Source: merckmillipore.com
Now you can calculate its boiling point under any pressure. 379 K Solubility in water. Therefore the organic layer could be above or below the aqueous layer depending on the organic solvent used. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. Immediate medical attention is required.
 Source: en.wikipedia.org
Source: en.wikipedia.org
2820 dynecm at 25 C. Methylene chloride 75-09-2 75 Ethanedioyl dichloride 79-37-8 25 4. Methylene chloride 95 75-09-2. Acetone CH 3 COCH 3. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
Source: chemspider.com
If you are not sure which layer is the organic or the aqueous layer perform the water drop test. 2806 kJmol at boiling point. 2820 dynecm at 25 C. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes.
Source:
19 mmHg 20C Hazards Safety data sheet. Add a drop of either layer on top of a watch glass filled with water. The sorption behavior of methylene blue and crystal violet from aqueous solution onto the sorbent was investigated under various experimental conditions. Having a normalized breakthrough time of 10 minutes or less. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles.
 Source: acs.org
Source: acs.org
Chloroform and methylene chloride are denser than water while most other organic solvents are not as dense as water. Therefore the organic layer could be above or below the aqueous layer depending on the organic solvent used. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. You can now roughly evaluate its boiling point. Methylene chloride is primarily used as a solvent in paint removers but is also used in aerosol formulations.
Source: stenutz.eu
Then extracted from the water with dichloromethane methylene chloride which is an organic solvent that is insoluble in water. Add a drop of either layer on top of a watch glass filled with water. 2806 kJmol at boiling point. If you are not sure which layer is the organic or the aqueous layer perform the water drop test. The main target users are workers and those responsible for occupational safety and health.
Industrially it is produced by the carbonylation of methylene chloride oxidation of vinylidene chloride or the addition of chlorine to ketene. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. 480 480 480 480 indicates greater than. Having a normalized breakthrough time of 10 minutes or less. But we want to separate the caffeine from the tannins by.
 Source: slideplayer.com
Source: slideplayer.com
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 50 ppm Vacated TWA. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. It has long been used in a variety of air-conditioning and refrigeration applications in a variety of markets. Hazardous Substances Data Bank HSDB 3220 Surface Tension.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It can however be cooled down to 78 C 108 F using a dry iceacetone bath Diethyl ether CH 3 CH 2 OCH 2 CH 3 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is. You can now roughly evaluate its boiling point. Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2. 106 C 223 F. Methylene chloride 95 75-09-2.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methylene chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.