Methanol boiling point celsius
Home » datasheet » Methanol boiling point celsiusMethanol boiling point celsius
Methanol Boiling Point Celsius. The ceramic electrolyte which can run as hot as 800 degrees Celsius has the advantage that the electrolyte stays solid. At 60 degrees Celsius most enzymes in liquid medium are inactivated. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The boiling point of water also depends on the purity of the water.
 Methanol From thermopedia.com
Methanol From thermopedia.com
Calculate its molar heat of vaporization. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. Pentane C 5 H 12. The melting point of formic acid is 84 degrees celsius whereas the boiling point of this compound corresponds to 1008 degrees celsius. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. The normal boiling point of dichloromethane is 40.
For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F.
It can also be noted that formic acid forms. Were talking saltier than the ocean levels of salt. 23 K to 353. This weak acid is known to form a miscible mixture with water. A polar solvent methanol acquired the name wood. Has weaker intermolecular forces and is a gas at 300 mmHg Part B.
 Source: thermopedia.com
Source: thermopedia.com
7837 C 1731 F Boiling point of nitrogen. Import numpy as np x nplinspace-nppi nppi 10 print x print x0 first element print x2 third element print x-1 last element print x-2 second to last element. 56 C 1328 F Boiling point of alcohol. Pentane C 5 H 12. Although adding salt to water raises its boiling point its worth noting the salted water actually boils more quickly.
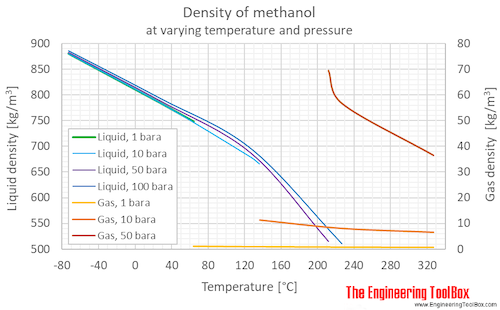 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water. 7837 C 1731 F Boiling point of nitrogen. Since both the methanol and the water remain as liquids only the specific heats for liquid will be involved in the calculation.
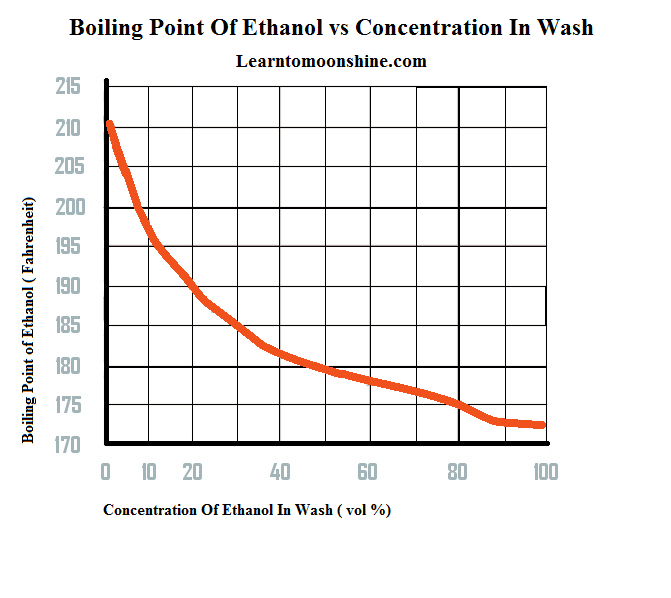 Source: learntomoonshine.com
Source: learntomoonshine.com
The conjugate base formed from the deprotonation of formic acid is commonly referred to as formate. At 60 degrees Celsius most enzymes in liquid medium are inactivated. To reach or cause something to reach the temperature at which a liquid starts to turn into a. Isopropanol Concentration by volume 0 10 20 30 40 50 60 70 80 90 100. They can deliver powers of several Megawatts but at a lower efficiency of around 35.
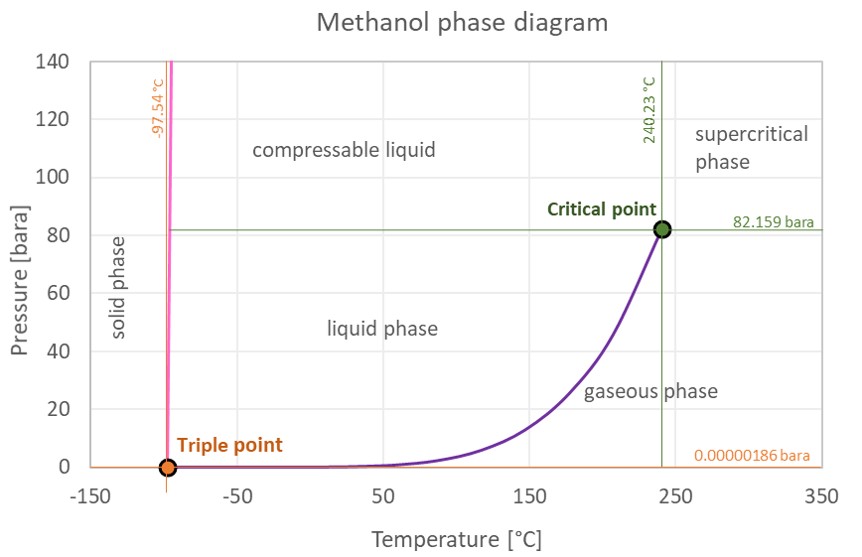 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
647 C 1485 F Boiling point of acetone. It can either change into a gas when it has reached its boiling point or it evaporates which is just when surface molecules of a liquid get j. The flash point of a chemical is the lowest temperature where it will evaporate enough. Use a heat source whose temperature can be quickly raised or lowered such as a heating mantle or bunsen burner but these may be hard to control the temperature. A key point to remember is that in python arrayvector indices start at 0.
 Source: researchgate.net
Source: researchgate.net
Thus alcohol evaporates into steam quicker than water. Import numpy as np x nplinspace-nppi nppi 10 print x print x0 first element print x2 third element print x-1 last element print x-2 second to last element. There is always a specific temperature of optimum activity of every enzyme which usually ranges. For example when it is reacted with hydrochloric acid ammonia is converted into ammonium chloride. 1 We look up the boiling point of methanol and find it to be 647 C.
 Source: researchgate.net
Source: researchgate.net
50 g of urea NH 2 CONH 2 is dissolved in 850 g of water. At that temperature water turns into water vapor or steam like you. At 60 degrees Celsius most enzymes in liquid medium are inactivated. 7837 C 1731 F Boiling point of nitrogen. When any liquid receives enough energy it starts to change its kinetic energy level and go from just a liquid to a liquid and some gas.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Energy Forms. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. Salting Water So It Boils Faster. 7837 C 1731 F Boiling point of methanol. It is a light volatile colourless flammable liquid with a distinctive alcoholic odour similar to that of ethanol potable alcohol.
 Source: ddbst.com
Source: ddbst.com
A key point to remember is that in python arrayvector indices start at 0. 7837 C 1731 F Boiling point of nitrogen. Although adding salt to water raises its boiling point its worth noting the salted water actually boils more quickly. All the salts that are produced from such acid-base reactions are known to contain the ammonium cation denoted by NH 4. Higher boiling point and has a higher heat of vaporization Substance B.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The boiling point temperature will be lower if the atmospheric pressure is decreased. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. It can either change into a gas when it has reached its boiling point or it evaporates which is just when surface molecules of a liquid get j. They can deliver powers of several Megawatts but at a lower efficiency of around 35. We can select a range of elements too.
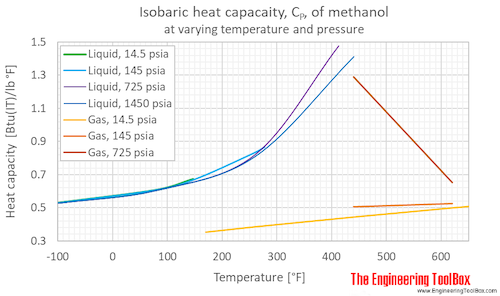 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
647 C 1485 F Boiling point of acetone. Freezing Point of Isopropanol 2-Propanol based Water Solutions. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Although adding salt to water raises its boiling point its worth noting the salted water actually boils more quickly. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methanol boiling point celsius by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.