Mercury 2 sulfate melting point
Home » datasheet » Mercury 2 sulfate melting pointMercury 2 sulfate melting point
Mercury 2 Sulfate Melting Point. Mercuric chloride is obtained by the action of chlorine on mercury or on mercuryI chlorideIt can also be produced by the addition of hydrochloric acid to a hot concentrated solution of mercuryI compounds such as the nitrate. 2 BaO O 2 2 BaO 2. The primary commercial source of barium is. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Mercury Sulfide Wikipedia From en.wikipedia.org
Mercury Sulfide Wikipedia From en.wikipedia.org
Cobalt sulfate is used in the electrochemical industries as a drier in paints and inks as a coloring agent in storage batteries and as a supplement for Vitamin B12 deficiency. 26 gcm-3 at 20C. 2 BaO O 2 2 BaO 2. Analytical 4 ACS reagent 2 BioReagent 2 Technique. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds such as hydrogen sulfide and ammonia.
Analytical 4 ACS reagent 2 BioReagent 2 Technique.
Strontium is a soft silver-yellow alkaline-earth metal. Electronic shell Kr 5s 2. 1000 K 727 C. Mercuric chloride is obtained by the action of chlorine on mercury or on mercuryI chlorideIt can also be produced by the addition of hydrochloric acid to a hot concentrated solution of mercuryI compounds such as the nitrate. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids. 0113 nm 2 Isotopes.
 Source: sciencemadness.org
Source: sciencemadness.org
Heating a mixture of solid mercuryII sulfate and sodium chloride also affords volatile HgCl 2. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. 0113 nm 2 Isotopes. In its solid form ice water is less. Energy of second ionisation.
 Source: americanelements.com
Source: americanelements.com
0113 nm 2 Isotopes. C At the pressure and temperature of point 1 Ys will spontaneously convert. Melting point - the temperature at which a solid turns into a liquid. Although its formula H 2 O seems simple water exhibits very complex chemical and physical properties. Analytical 4 ACS reagent 2 BioReagent 2 Technique.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
According to the phase diagram given for Compound Y what description is correct. Substance Formula Melting point C Boiling temperature C Density 25C. 26 gcm-3 at 20C. Energy of first ionisation. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds such as hydrogen sulfide and ammonia.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Exposure to cobalt sulfate results in irritation of the skin eyes and respiratory tract and affects the thyroid lungs heart and kidneys. The primary commercial source of barium is. A 1 2 and 3 b 4 5 and 6 c 1 and 2 only d 4 and 6 only e some other combination 11. 1000 K 727 C. This agent may also inhibit acid production by commensal oral bacteria.

Strontium is a soft silver-yellow alkaline-earth metal. Strontium is a soft silver-yellow alkaline-earth metal. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds such as hydrogen sulfide and ammonia. 2 Its melting temperatures of 1000 K 730 C. A At the temperature and pressure at point 4 Yg will spontaneously convert to Ylb At 0 o C and 1200 torr Y exists as a solid.
 Source: onyxmet.com
Source: onyxmet.com
Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. 0113 nm 2 Isotopes. A 1 2 and 3 b 4 5 and 6 c 1 and 2 only d 4 and 6 only e some other combination 11. Cobalt sulfate is used in the electrochemical industries as a drier in paints and inks as a coloring agent in storage batteries and as a supplement for Vitamin B12 deficiency. In its solid form ice water is less.
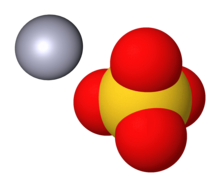 Source: en.wikipedia.org
Source: en.wikipedia.org
Strontium is a soft silver-yellow alkaline-earth metal. 1000 K 727 C. Heating a mixture of solid mercuryII sulfate and sodium chloride also affords volatile HgCl 2. According to the phase diagram given for Compound Y what description is correct. Cobalt sulfate is used in the electrochemical industries as a drier in paints and inks as a coloring agent in storage batteries and as a supplement for Vitamin B12 deficiency.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Energy of first ionisation. 2 BaO O 2 2 BaO 2. In its solid form ice water is less. Heating a mixture of solid mercuryII sulfate and sodium chloride also affords volatile HgCl 2. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids.
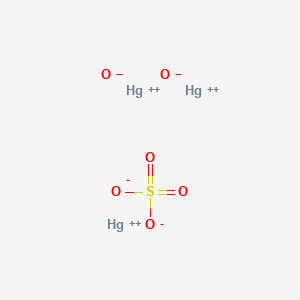
0113 nm 2 Isotopes. Barium sulfate was first applied as a radiocontrast agent in X-ray imaging of the digestive system in 1908. Energy of first ionisation. 2 BaO O 2 2 BaO 2. A At the temperature and pressure at point 4 Yg will spontaneously convert to Ylb At 0 o C and 1200 torr Y exists as a solid.
 Source: wikiwand.com
Source: wikiwand.com
26 gcm-3 at 20C. Hg 2 NO 3 2 4 HCl 2 HgCl 2 2 H 2 O 2 NO 2. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. This agent may also inhibit acid production by commensal oral bacteria. Boiling Point C Feature.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mercury 2 sulfate melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.