Melting points of benzene
Home » datasheet » Melting points of benzeneMelting points of benzene
Melting Points Of Benzene. Eon Pommier et al 1971. Thats not really key to the discussion. Pyridine has a lower symmetry than benzene hence its lower melting point but the melting point again increases with diazine and triazines. Youngs Modulus of Elasticity.
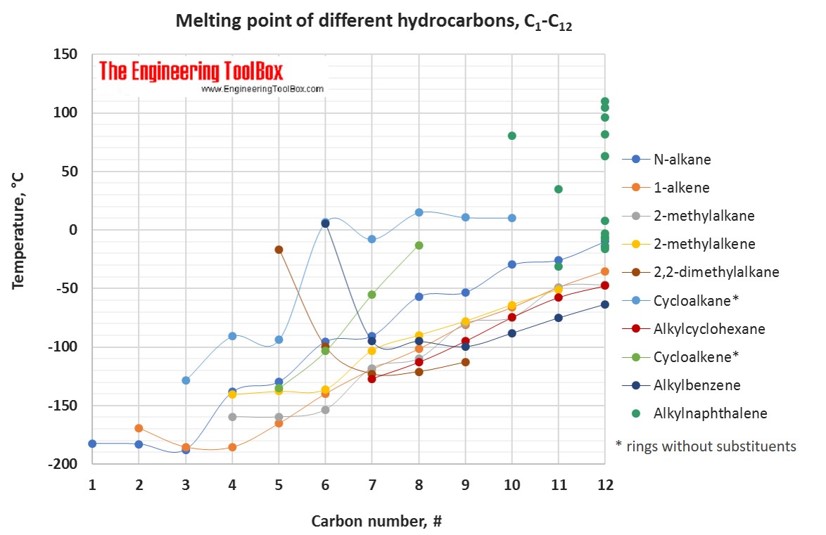 Melting Points Of Hydrocarbons Alcohols And Acids From engineeringtoolbox.com
Melting Points Of Hydrocarbons Alcohols And Acids From engineeringtoolbox.com
Its melting and boiling points are 553 C and 801 C respectively. Linear versus branched — higher meltingboiling points due to better stacking and surface area contact. Pyridine has a lower symmetry than benzene hence its lower melting point but the melting point again increases with diazine and triazines. A pure solid material melts at specific temperature. A high melting point results from a high heat of fusion a low entropy of fusion or a combination of both. But once you move beyond benzene.
In highly symmetrical molecules the.
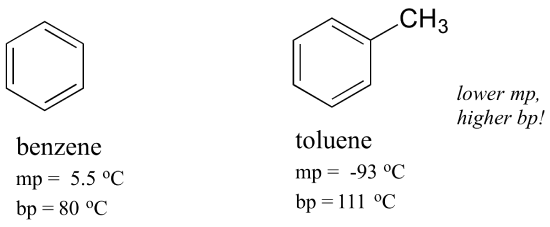
Thats not really key to the discussion. The formula for benzene the simplest arene and base structure for all others is. But once you move beyond benzene. The benzene layer was separated and the aqueous layer extracted with four 25 ml portions of benzene. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the cumulative strength of intermolecular London dispersion forces. 1084 C 1983 F Melting point of iron.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
A Need H bonded to O N or F. So para isomer is more symmetric than ortho and meta. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. B CHF 3 CH 3 COCH 3 acetone. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the cumulative strength of intermolecular London dispersion forces.
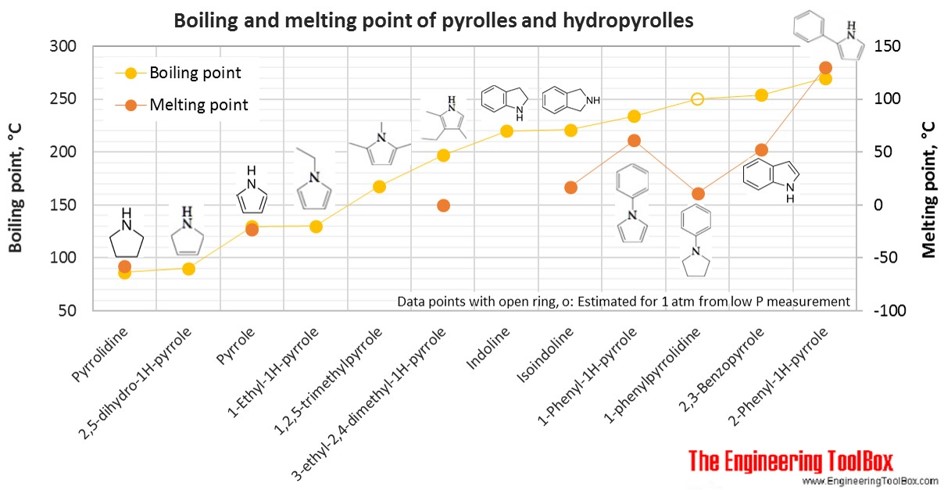 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
However care must be taken when dealing with drugs with relatively low melting point or when excipients with relatively low melting points are included in a solid formulation. The benzene layer was separated and the aqueous layer extracted with four 25 ml portions of benzene. But how does this ortho para affect the difference in boiling points and melting points when it comes to isomerism. June 10 2019 at 947 pm. The melting point is also referred to as liquefaction point solidus or liquidus.
 Source: clutchprep.com
Source: clutchprep.com
Pure substances melt at a sharp highly-defined temperature very small temperature range of 05 1 C whereas impure contaminated substances generally exhibit a large melting interval. The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier sectionThe chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene. 1538 C 2800 F Melting point of lead. B CHF 3 CH 3 COCH 3 acetone. Linear versus branched — higher meltingboiling points due to better stacking and surface area contact.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Notice that the boiling points of the unbranched alkanes pentane through decane increase rather smoothly with molecular weight but the melting points of the even-carbon chains increase more than those of the odd-carbon chains. Melting Point-15C Molecular Weight. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the cumulative strength of intermolecular London dispersion forces. 961 C 1761 F. So para isomer is more symmetric than ortho and meta.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
Boiling Points of Benzene Ethylene Chloride n-Heptane and 224-Trimethylpentane. So para isomer is more symmetric than ortho and meta. The benzene was distilled off and the remaining viscous oil was distilled under reduced pressure. Hi there Is -CH₂OH an Ortho Para director or Meta Director. If you try to boil water at a high.
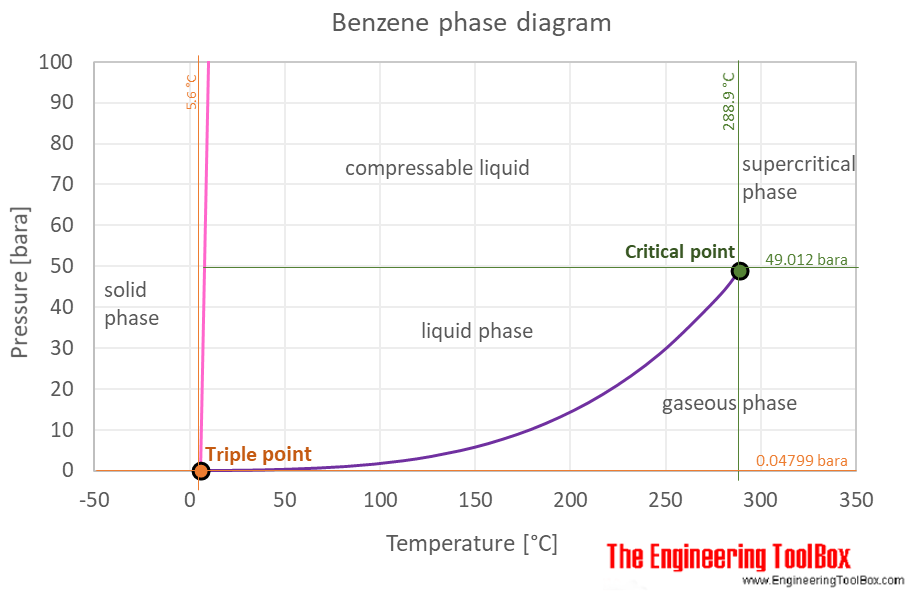 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Hi there Is -CH₂OH an Ortho Para director or Meta Director. Hi there Is -CH₂OH an Ortho Para director or Meta Director. The temperature at which all material of a contaminated substance is molten is. 1538 C 2800 F Melting point of lead. December 4 2018 at 634 am.
 Source: bartleby.com
Source: bartleby.com
University of California Davis. The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier sectionThe chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon. A Need H bonded to O N or F. Pyridine has a lower symmetry than benzene hence its lower melting point but the melting point again increases with diazine and triazines.
 Source: researchgate.net
Source: researchgate.net
Coefficents calculated by NIST from authors data. Pure substances melt at a sharp highly-defined temperature very small temperature range of 05 1 C whereas impure contaminated substances generally exhibit a large melting interval. 1425-1540 C 2600-2800 F Melting point of gold. 1064 C 19475 F Melting point of copper. Hi there Is -CH₂OH an Ortho Para director or Meta Director.
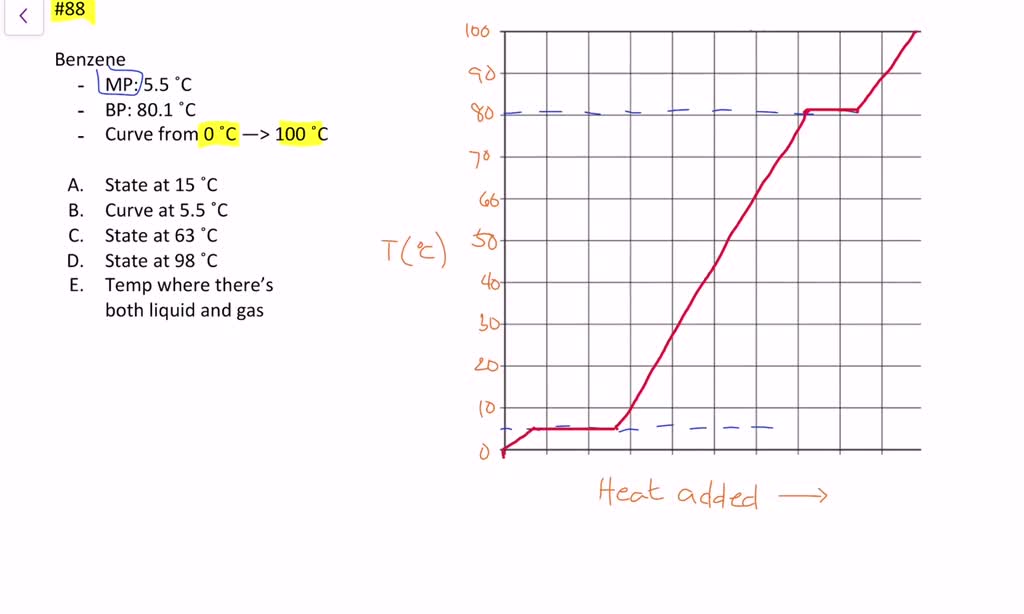 Source: numerade.com
Source: numerade.com
Viscosity - Documents giving viscosity of different kind of chemical species at varying conditions. Pure substances melt at a sharp highly-defined temperature very small temperature range of 05 1 C whereas impure contaminated substances generally exhibit a large melting interval. Hydrogen bonds are weaker than covalent bonds but stronger than b or c below. Also freezing melting and condensation points can change for different substances depending on the pressure they are under. Micro-boiling Point Measurement.
 Source: ch.imperial.ac.uk
Source: ch.imperial.ac.uk
Melting points are often used to characterize organic and inorganic crystalline compounds and to ascertain their purity. Hi there Is -CH₂OH an Ortho Para director or Meta Director. Even-membered chains pack together in a uniform fashion more compactly than do odd-membered chains. Melting points of common materials Melting point of steel. Fortunately the melting points of majority of the APIs and excipients for solid oral formulations are well above the typical operating temperature range for APS testing 4080C.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting points of benzene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.