Melting point vanadium
Home » datasheet » Melting point vanadiumMelting point vanadium
Melting Point Vanadium. Melting points for some. Energy of third ionisation. 2 - Atomic number-259. In general melting is a phase change of a substance from the solid to the liquid phase.
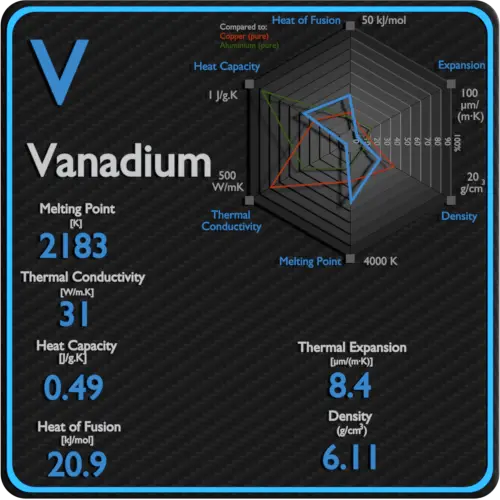 Vanadium Thermal Properties Melting Point Thermal Conductivity Expansion From material-properties.org
Vanadium Thermal Properties Melting Point Thermal Conductivity Expansion From material-properties.org
Description Your user agent does not support the HTML5 Audio element. Reference Kelvin Celsius Fahrenheit Comments 1 H hydrogen H 2 use. When heated carbon undergoes a phase change directly from solid to gas. Melting point of tool steel A2 steel is around 1420C. Because of its excellent physical and. Andrés Manuel del Río discovered compounds.
Vanadium is a silver-gray metal with a high melting point and is often called refractory metal together with niobium tantalum tungsten and molybdenum.
Energy of fourth ionisation. The melting point or rarely liquefaction point of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Tungsten takes the 4th spot in our list of the materials with the highest melting point. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Therefore zinc does not contribute much electrons to the metallic lattice like other 3d metals. Energy of fourth ionisation.
Source: theodoregray.com
Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. 2183 K 1910 C. Melting Point of Tool Steel A2 Steel. Vanadium is a silver-gray metal with a high melting point and is often called refractory metal together with niobium tantalum tungsten and molybdenum. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications.
Source: periodictable.com
Adding a heat will convert the solid into a liquid with no temperature change. Melting Point C F Admiralty Brass. However other factors–such as crystal structure atomic weight and electron structure–can also influence the melting point. Melting point is the temperature at which a substance changes from solid to liquid state. Value given for alpha form.
 Source: material-properties.org
Source: material-properties.org
Symbols Melting Point Name 095 K-27205 C-458 F. Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. However other factors–such as crystal structure atomic weight and electron structure–can also influence the melting point. Cast Iron Gray 1175-1290. Let us look at the elements in the ascending order of their melting points.
 Source: thoughtco.com
Source: thoughtco.com
The melting point of a substance depends on pressure and is usually specified at standard pressure. The chemical elements of the periodic chart sorted by. Because of its excellent physical and. The melting point of a substance depends on pressure and is usually specified at standard pressure. Boiling point The temperature at which the liquidgas phase change occurs.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It has better gas salt and water corrosion resistance than most stainless steels. The elements of the periodic table sorted by melting point. 0074 nm 3. 24553 K-248447 C-415205 F. However other factors–such as crystal structure atomic weight and electron structure–can also influence the melting point.
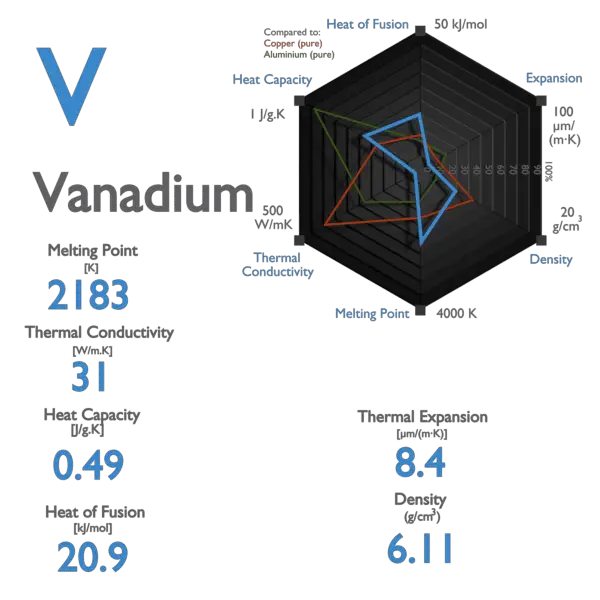 Source:
Source:
Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Melting points for some. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. When heated carbon undergoes a phase change directly from solid to gas. Vanadium is a chemical element with the symbol V and atomic number 23.

Moreover vanadium is malleable hard non-magnetic and has the ability to resist hydrochloric acid and sulfuric acid. 14025 K-258975 C-434 F. Value given for diamond form. Therefore zinc does not contribute much electrons to the metallic lattice like other 3d metals. At the melting point the solid and liquid phases exist in equilibrium.
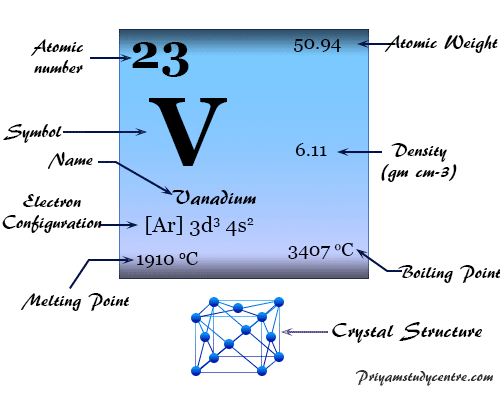 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Andrés Manuel del Río discovered compounds. Description Your user agent does not support the HTML5 Audio element. Value given for diamond form. The 38 elements in groups 3 through 12 of the periodic table are called transition metals. At normal atmospheric pressure carbon does not melt when heated it sublimes.
 Source: britannica.com
Source: britannica.com
Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Melting Point of Tool Steel A2 Steel. However other factors–such as crystal structure atomic weight and electron structure–can also influence the melting point. The melting point of a substance depends on pressure and is usually specified at standard pressure. The melting point of a substance is the temperature at which this phase change occurs.
 Source: onyxmet.com
Source: onyxmet.com
If the pressure is increased to 10 atmospheres. When heated carbon undergoes a phase change directly from solid to gas. The melting point of a material is primarily related to bond strength. Nils Sefstrom in 1830. Boiling point The temperature at which the liquidgas phase change occurs.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point vanadium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.