Melting point sodium bicarbonate
Home » datasheet » Melting point sodium bicarbonateMelting point sodium bicarbonate
Melting Point Sodium Bicarbonate. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. 2159 NTP 1992 Boiling Point. When using the potato.
 Baking Soda Molecular Formula Sodium Bicarbonate From thoughtco.com
Baking Soda Molecular Formula Sodium Bicarbonate From thoughtco.com
In the year 1846 Austin Church and John Dwight bakers of New York started the first factory to produce baking soda. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. Sodium oxide reacts with carbon dioxide to form. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. The mixture is dissolved in ether and mixed thoroughly with aqueous sodium bicarbonate weaker base. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming.
Both the melting and boiling point of sodium are quite high.
A strong base such as sodium hydroxide is not necessary in this particular case. 882940C 1621292F 1156090 K Block. Examples of the uses of sodium bicarbonate include baking as a raising agent and sodablasting. 6 teaspoons of Sodium sulfate 4. 2159 NTP 1992 Boiling Point. Note that instead of using sodium hydroxide as the base sodium bicarbonate is used.
 Source: thoughtco.com
Source: thoughtco.com
Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda NaHCO 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry-chemical fire extinguishers. The corrosion of solid sodium by oxygen also is. When you heat it it forms sodium carbonate. Sodium oxide reacts with carbon dioxide to form. Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOH.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Baking soda actually contains sodium its in the name and its chemical name is sodium bicarbonate where Im sure youve come across it in baking or cooking where it undergoes thermal decomposition at above 70C to release carbon dioxide - which then makes your. LibriVox is a hope an experiment and a question. When you heat it it forms sodium carbonate. Put 15 mL of coffee and tea solution in a separatory funnel 3. Nicolas Leblanc a French chemist produced sodium carbonate in the year 1791.
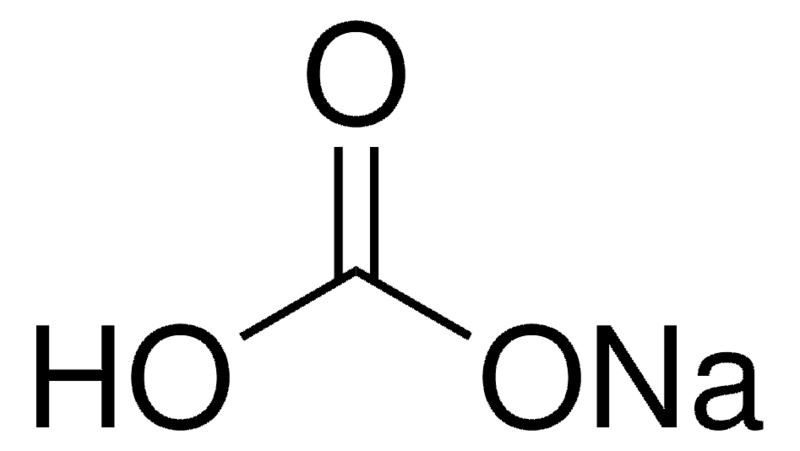 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It is a white solid crystalline chemical compound usually in its powder form. 5 hydrochloric acid 5 sodium hydroxide solution saturated sodium bicarbonate solution 6 and water. The mixture is dissolved in ether and mixed thoroughly with aqueous. 2159 NTP 1992 Boiling Point. Can the net harness a bunch of volunteers to help bring books in the public domain to life through podcasting.
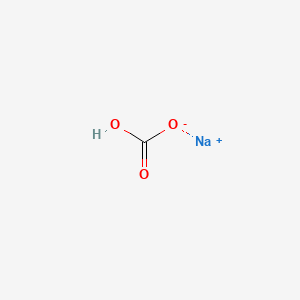
The dominant yellow component. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc. Decomposes at 228 F NTP 1992 Vapor Pressure. More concentrated solutions are rarely used for extraction because of the increased evolution. Sodium is a good conductor of electricity which means that electric current can pass through this element without much resistance.

Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. Baking soda actually contains sodium its in the name and its chemical name is sodium bicarbonate where Im sure youve come across it in baking or cooking where it undergoes thermal decomposition at above 70C to release carbon dioxide - which then makes your. Set a separatory funnel 2. This agent may also inhibit acid production by commensal oral bacteria. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc.
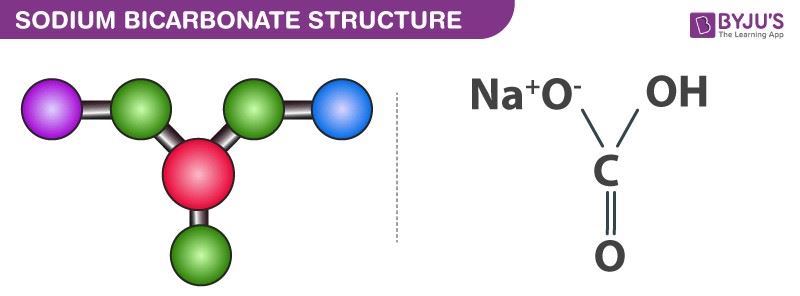 Source: byjus.com
Source: byjus.com
Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. It is a white solid crystalline chemical compound usually in its powder form. 6 teaspoons of Sodium sulfate 4. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. The ether layer is then extracted with sodium hydroxide stronger base the layers separated.
 Source: in.pinterest.com
Source: in.pinterest.com
LibriVox is a hope an experiment and a question. Will be purified by recrystallization and identified by melting points. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. Along with potassium many important medicines have sodium added to improve their bioavailability. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda NaHCO 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry-chemical fire extinguishers.
 Source: slideplayer.com
Source: slideplayer.com
Sodium chloride is extensively used for anti-icing and de-icing and as a preservative. Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. The ether layer is then extracted with sodium hydroxide stronger base the layers separated. Sodium bicarbonate or Sodium hydrogen carbonate has a monoclinic crystalline structure. The mixture is dissolved in ether and mixed thoroughly with aqueous sodium bicarbonate weaker base.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
7295 JmolK Hydrogen Bond Acceptor. 6 teaspoons of Sodium sulfate 4. Abundant sodium is found in the sun and stars. After the layers settle they are separated and placed into different tubes. Though potassium is the better ion in most cases sodium is chosen for its lower price and atomic weight.
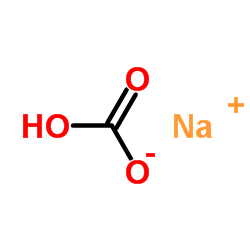 Source: chemsrc.com
Source: chemsrc.com
Decomposes at 228 F NTP 1992 Vapor Pressure. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. Add two pinches per ounce of clean jewelry scrap and more for dirty scrap. 97794C 208029F 370944 K Period 3 Boiling point. Abundant sodium is found in the sun and stars.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point sodium bicarbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.