Melting point range of benzoic acid
Home » datasheet » Melting point range of benzoic acidMelting point range of benzoic acid
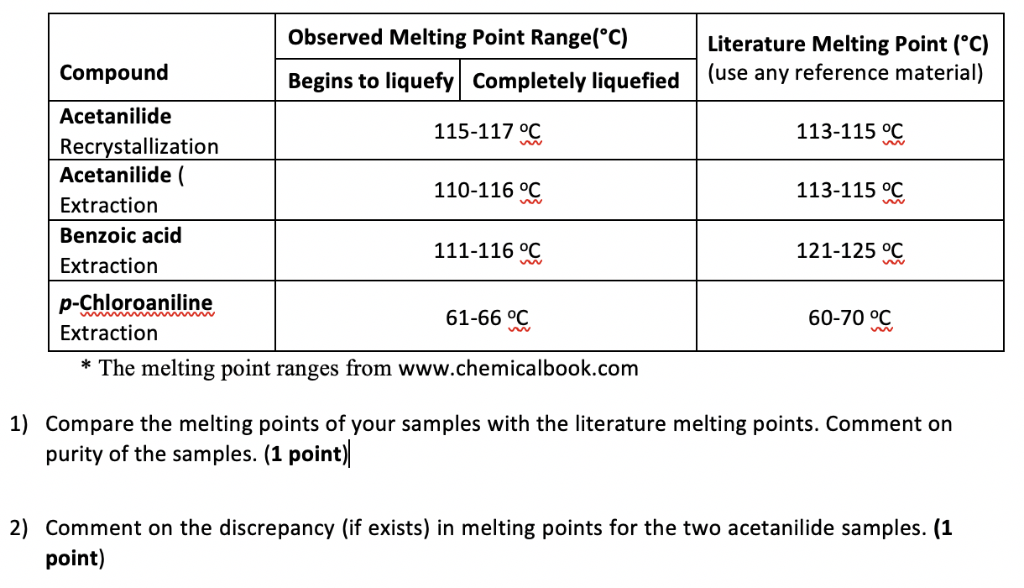
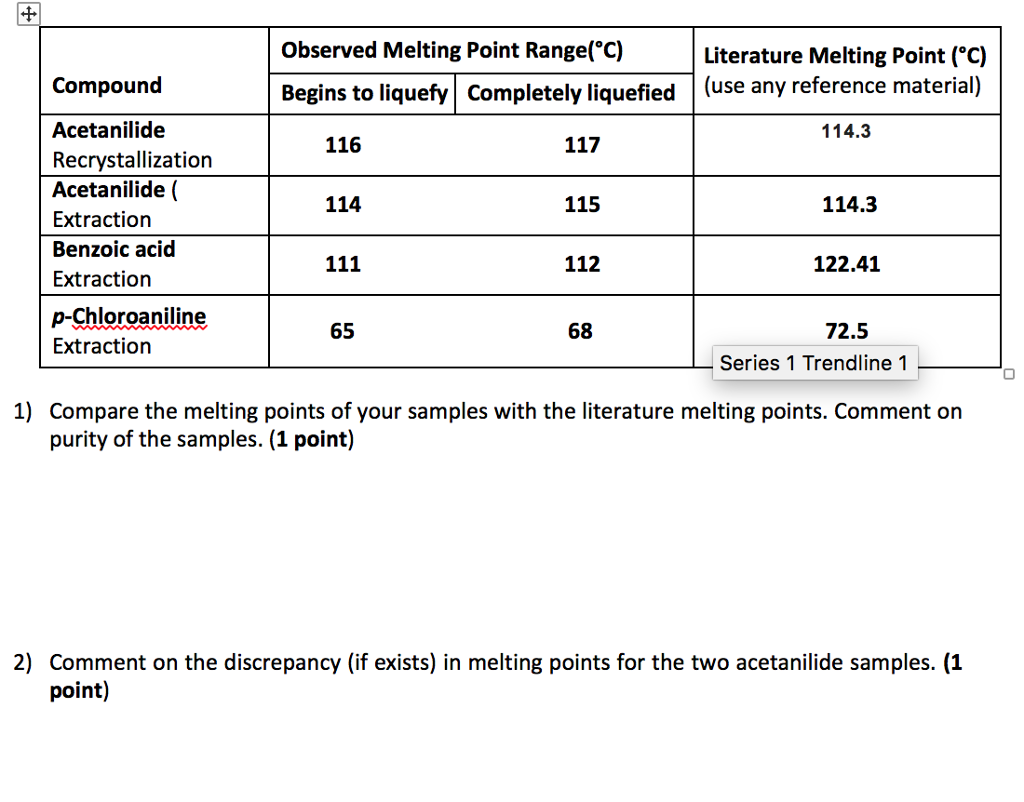
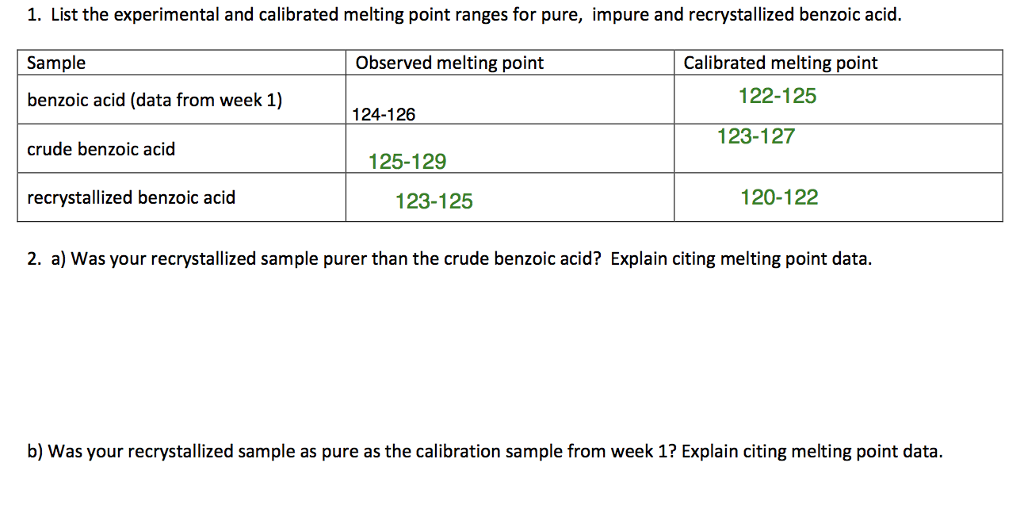
Melting Point Range Of Benzoic Acid. Dip the open end of the capillary tube in the finely powdered Benzoic acid. For example if A is cinnamic acid mp. A solid compound changes to a. The melting points of the recrystallized benzoic acid were 114- 122 C and 121-127 C both encompassing the expected value and allowing one to believe that the sample was close to pure which was the goal of recrystallization.
 Melting Point Standard 121 123 C Analytical Standard 65 85 0 From sigmaaldrich.com
Melting Point Standard 121 123 C Analytical Standard 65 85 0 From sigmaaldrich.com
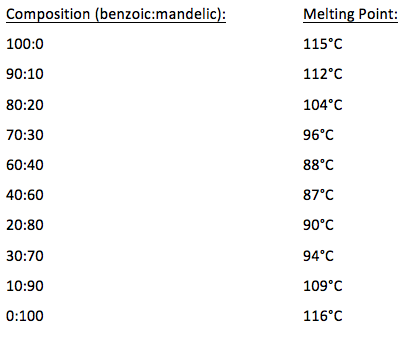
The first experiment will give you practice in the technique as you recrystallize benzoic acid from water. Benzoic acid and naphthalene. A sharp melting point is often evidence that a sample is fairly pure and a wide melting range is evidence that it is not pure. Melting point range for your unknown compound 3 the compound names and melting ranges for your mixture melting experiments and 4 the name structure and melting point range of the known compound that most clearly agrees with your measured melting point. If benzoic were contaminated with an impurity the melting point range might decrease and broaden to 117-120 C. The lowest mixture melting point e is called the eutectic point.
Also benzoic acid and its salts are widely used in the food industry as food preservatives.
4649C Flash point. Recrystallization and Melting Point. Glycolic acid is found in some sugar-crops. The melting point range for pure solids is narrow usually only 1 to 2 degrees Celsius known as a sharp melting point. Aspirin an acetyl derivative of salicylic acid is a white crystallize weakly acidic substance with a melting point of 136oC and a boiling point of 140oC Meyers 2007. A Well below the melting point b Glistening c First liquid droplet seen d Sample is completely melted.
 Source: chegg.com
Source: chegg.com
The purer the sample the narrower the melting point range. Modern instruments like the Melting Point Excellence instruments by METTLER TOLEDO enable automated detection of the melting point and melting range and visual inspection by a video camera. Of these compounds only salicylate and p-nitrobenzoic acid were not activated to their respective CoA esters. Melting point range for your unknown compound 3 the compound names and melting ranges for your mixture melting experiments and 4 the name structure and melting point range of the known compound that most clearly agrees with your measured melting point. An esterification reaction is the general name for a chemical reaction in which two reactants typically an alcohol and an acid form an ester as the.
 Source: lgcstandards.com
Source: lgcstandards.com
Use of apparatus and techniques. 4649C Flash point. Additional pictures for the melting of this sample can be seen in Figure 62. If the melting. Benzoic Acid 1213 Found naturally in berries 122-123 Urea 2122 Used as fertilizer 132-133 Cinnamic Acid 1113 Oxidation product of cinnamon oil 132-133 Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154.
 Source: chegg.com
Source: chegg.com
Balmer 2 Experimental Procedure Fig1. If the compound melts over a very narrow range it can usually be assumed that the compound is relatively pure. A small amount of compound B in a sample of compound A lowers and broadens its melting point. 1224 deg C Solubilities. With the Melting Point Excellence instruments by METTLER TOLEDO up to 6 capillaries.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Of these compounds only salicylate and p-nitrobenzoic acid were not activated to their respective CoA esters. 1224 deg C Solubilities. The first experiment will give you practice in the technique as you recrystallize benzoic acid from water. For a material whose identity is known an estimate of degree of purity can be made by comparing its melting point with that of a pure sample. There are three main reasons to record the melting point of a.

The first experiment will give you practice in the technique as you recrystallize benzoic acid from water. Hold the closed end of the capillary tube between your finger and thumb as shown in figure a above. Function and uses Benzoic acid is a natural ingredient occurring in many foodstuffs and in plant extracts. Melting point range for your unknown compound 3 the compound names and melting ranges for your mixture melting experiments and 4 the name structure and melting point range of the known compound that most clearly agrees with your measured melting point. Melting of benzoic acid.
 Source: chegg.com
Source: chegg.com
The melting point of benzoic acid is approximately 122 C 2002 Gilbert and Martin CD-Rom MSDS Data. 4649C Flash point. It can be noted that the ideal temperature under which this process can be carried out is in the range of 300 to 400 degrees celsius. The purer the sample the narrower the melting point range. The capillary method is required in many local pharmacopeias as the standard technique for melting point determination.

Balmer 2 Experimental Procedure Fig1. Benzoic acid 419 Acetic acid 475 water 140 Acid pKa. Benzoic Acid Created by Global Safety Management Inc. Benzoic Acid 1213 Found naturally in berries 122-123 Urea 2122 Used as fertilizer 132-133 Cinnamic Acid 1113 Oxidation product of cinnamon oil 132-133 Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154. A small amount of compound B in a sample of compound A lowers and broadens its melting point.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The first experiment will give you practice in the technique as you recrystallize benzoic acid from water. Chemical formula C 2 H 4 O 3 also written as HOCH 2 CO 2 H is the smallest α-hydroxy acid AHA. A solid compound changes to a. A Well below the melting point b Glistening c First liquid droplet seen d Sample is completely melted. 34 gl 25C Boiling pointBoiling range.
 Source: odinity.com
Source: odinity.com
Mandatory experiment 71 - Recrystalisation of benzoic acid and determination of its melting point. Benzoic Acid Created by Global Safety Management Inc. Salicylate p-chloro- and p-nitrobenzoic acids effectively. Using a hot plate dissolve approximately 10 g of impure benzoic acid in 30 35 mL of hot water water at or near its bp in a 125 mL Erlenmeyer flask. The melting points of the recrystallized benzoic acid were 114- 122 C and 121-127 C both encompassing the expected value and allowing one to believe that the sample was close to pure which was the goal of recrystallization.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
34 gl 25C Boiling pointBoiling range. Using a hot plate dissolve approximately 10 g of impure benzoic acid in 30 35 mL of hot water water at or near its bp in a 125 mL Erlenmeyer flask. The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. For a material whose identity is known an estimate of degree of purity can be made by comparing its melting point with that of a pure sample. Chemical formula C 2 H 4 O 3 also written as HOCH 2 CO 2 H is the smallest α-hydroxy acid AHA.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point range of benzoic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.