Melting point range of acetylsalicylic acid
Home » datasheet » Melting point range of acetylsalicylic acidMelting point range of acetylsalicylic acid
Melting Point Range Of Acetylsalicylic Acid. Aspirin is a trade name for acetylsalicylic acid a common analgesic. In the real world however. Dont heat too fast. Aspirin is a derivative of salicylic.
 Acetylsalicylic Acid By Jeraun Pogue Tierra Dixon Chemistry Ll Mrs Hall October 27 Ppt Download From slideplayer.com
Acetylsalicylic Acid By Jeraun Pogue Tierra Dixon Chemistry Ll Mrs Hall October 27 Ppt Download From slideplayer.com
Aspirin is a trade name for acetylsalicylic acid a common analgesic. Range of the melting point indicate the purity of the compound. Not Determined Evaporation rate. 4318 K Boiling point. Acetylsalicylic Acid ASA The synthesis of ASA from salicylic acid results in the formation of an ester functional group and therefore is called an esterification. The therapeutic efficacy of Acetylsalicylic acid can be decreased when used in combination with Mesalazine.
Then determine the melting point of aspirin using necessary apparatus.
Aspirin is a derivative of salicylic. Acetylsalicylic Acid ASA The synthesis of ASA from salicylic acid results in the formation of an ester functional group and therefore is called an esterification. If impurities are present in your crude sample the melting point range for your product will be lower than the range of pure aspirin. Not Determined Decomposition temperature. An ester has replaced the acidic phenol in ASA. A useful synthesis of acetylsalicylic acid was developed in 1893 patented in 1899 marketed under the trade name of aspirin by the Bayer Company in Germany.
 Source: odinity.com
Source: odinity.com
Mesalazine may decrease the excretion rate of Acrivastine which could result in a higher serum level. We own and operate 500 peer-reviewed clinical medical life sciences engineering and management journals and hosts 3000 scholarly conferences per year in the fields of clinical medical pharmaceutical life sciences business engineering and. 1586 C 3175 F. DIGITIZED BY COBLENTZ SOCIETY BATCH I FROM HARD COPY. 1600g of aspirin was obtained after the second synthesis.
 Source: researchgate.net
Source: researchgate.net
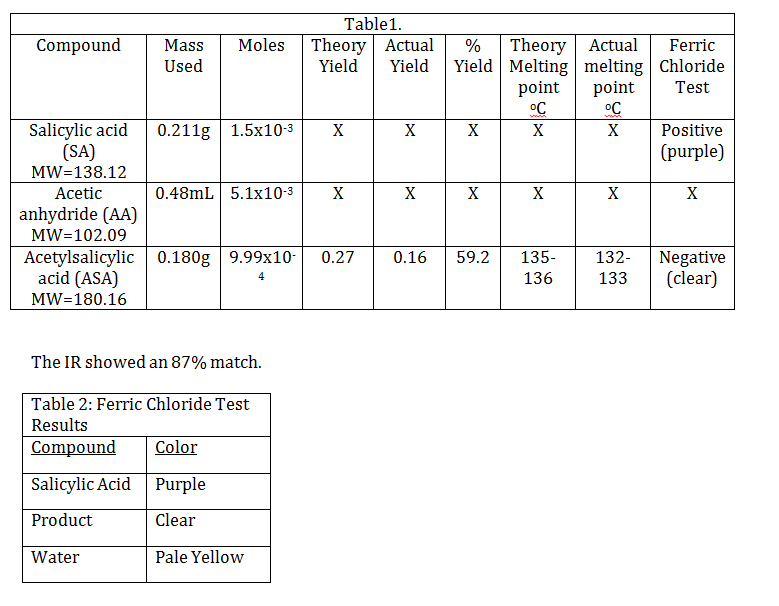
Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154 Mannitol 0201 Manufacture of radio condensers 167-170 Tartaric Acid 1111 In soft drinks cream of tartar 168-170 Itaconic Acid 0112 A dicarboxylic acid 166. 134 - 136 C 273 - 277 F Solubilities. It was thin short white crystals and had a melting point range or 154-155 degrees Celsius. DIGITIZED BY COBLENTZ SOCIETY BATCH I FROM HARD COPY. 2-Ethanoyloxybenzenecarboxylic acid He called the new medicine aspirin a for acetyl the systematic name for the compound at the time was acetylsalicylic acid spir for spirea the meadowsweet plant.
 Source: researchgate.net
Source: researchgate.net
Bismuth subsalicylate a salt of bismuth and salicylic acid is the active ingredient in stomach-relief aids such as Pepto-Bismol is the main. In Part I of this. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. 134 - 136 C 273 - 277 F Solubilities. 1081g of Salicylic acid was obtained after the first synthesis.
 Source: docsity.com
Source: docsity.com
1586 C 3175 F. Also your melting point range may be greater than 2 degrees. The Carboxylic acid COOH group on the Aspirin allows molecules of aspirin to form hydrogen bonds with each other see hydrogen bonding in aspirin section. The first step in this. Doses of ursodeoxycholic acid in the range of 16-20 mgkgday have been tolerated for 6-37 months without symptoms by 7 patients.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin. This shows that the impure aspirin sample contains acetylsalicylic acid. 25 GL 15C Boiling pointBoiling range. This was a white powder and had a melting point range of 134-135 degrees. In the real world however.
 Source: slideplayer.com
Source: slideplayer.com
A large melting point range would suggest an impure sample and ineffective recrystallization process. Aspirin is a trade name for acetylsalicylic acid a common analgesic. Aspirin is a derivative of salicylic. The risk or severity of. Acetylsalicylic Acid ASA The synthesis of ASA from salicylic acid results in the formation of an ester functional group and therefore is called an esterification.
 Source: packerintersections.com
Source: packerintersections.com
Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154 Mannitol 0201 Manufacture of radio condensers 167-170 Tartaric Acid 1111 In soft drinks cream of tartar 168-170 Itaconic Acid 0112 A dicarboxylic acid 166. 134 - 136 C 273 - 277 F Solubilities. PERKIN-ELMER 21 GRATING Instrument parameters. To prove purity you would need to resort to different analytic methods such as melting point historically HPLC-MS or GC-MS NMR etc. The therapeutic efficacy of Acetylsalicylic acid can be decreased when used in combination with Mesalazine.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
DIGITIZED BY COBLENTZ SOCIETY BATCH I FROM HARD COPY. Then determine the melting point of aspirin using necessary apparatus. Tryptophan is the least plentiful of all 22 amino acids and an essential amino acid in humans provided by food Tryptophan is found in most proteins and a precursor of serotoninTryptophan is converted to 5-hydroxy-tryptophan converted in turn to serotonin a neurotransmitter essential in regulating appetite sleep mood and painTryptophan is a natural sedative and present in dairy. A useful synthesis of acetylsalicylic acid was developed in 1893 patented in 1899 marketed under the trade name of aspirin by the Bayer Company in Germany. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year.
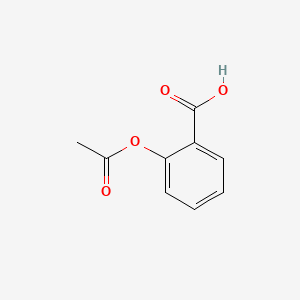
A useful synthesis of acetylsalicylic acid was developed in 1893 patented in 1899 marketed under the trade name of aspirin by the Bayer Company in Germany. Tryptophan is the least plentiful of all 22 amino acids and an essential amino acid in humans provided by food Tryptophan is found in most proteins and a precursor of serotoninTryptophan is converted to 5-hydroxy-tryptophan converted in turn to serotonin a neurotransmitter essential in regulating appetite sleep mood and painTryptophan is a natural sedative and present in dairy. 2-Ethanoyloxybenzenecarboxylic acid He called the new medicine aspirin a for acetyl the systematic name for the compound at the time was acetylsalicylic acid spir for spirea the meadowsweet plant. Determine start and end temperature of melting. Then determine the melting point of aspirin using necessary apparatus.
 Source: manualzz.com
Source: manualzz.com
134 - 136 C 273 - 277 F Solubilities. More than 50 million 5-grain tablets of aspirin are consumed daily in the United States. Therefore it is pure. A small melting point range close to the standard of acetylsalicylic acid would suggest a high purity sample meaning the recrystallization process was effective. From the titration of your sample the moles of acetylsalicylic.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point range of acetylsalicylic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.