Melting point of tellurium
Home » datasheet » Melting point of telluriumMelting point of tellurium
Melting Point Of Tellurium. At normal atmospheric pressure arsenic does not melt when heated. Value given for monoclinic beta form. Adding a heat will convert the solid into a liquid with no temperature change. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
 Cqf9rikabw6qam From
Cqf9rikabw6qam From
Thus higher the stronger the bond between the atoms higher will be the melting point. Adding a heat will convert the solid into a liquid with no temperature change. Tellurium Te has the highest melting point and boiling point. As a result the enriched target material irradiation was introduced to the MARIA Research. 85 - Boiling point. 5 - Inventor surname.
The elements of the periodic table sorted by melting point.
It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. At the melting point the solid and liquid phases exist in equilibrium. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. Boiling Point of all the elements in the Periodic Table in Graph and Table format Complete information about all the properties of elements using Graphs and Tables Interactive Dynamic Periodic Table Periodic Table Element Comparison Element Property trends and complete information about the element - Facts How to Locate on Periodic Table History Abundance Physical Properties Thermal. According to the analysis of surface energy the Te nanowires.
 Source: slideplayer.com
Source: slideplayer.com
In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. 79 - Vanderwaals radius. 7837 C 1731 F Boiling point of methanol. 85 - Boiling point. Tellurium is far more common in the Universe as a whole than on Earth.
 Source:
Source:
3732 K Boiling point of ethanol. 7837 C 1731 F Boiling point of nitrogen. Melting point of Thorium. Melting Point Boiling Point Date of Discovery Crystal Structure. 85 - Boiling point.
 Source: britannica.com
Source: britannica.com
Boiling points increases on moving down from fluorine to iodine. Tellurium dioxide shall not reach the melting point during the irradiation due to safety reasons. Boiling points of group 17. Value given for yellow phosphorus form. Lower Explosive Limit.
 Source: dreamstime.com
Source: dreamstime.com
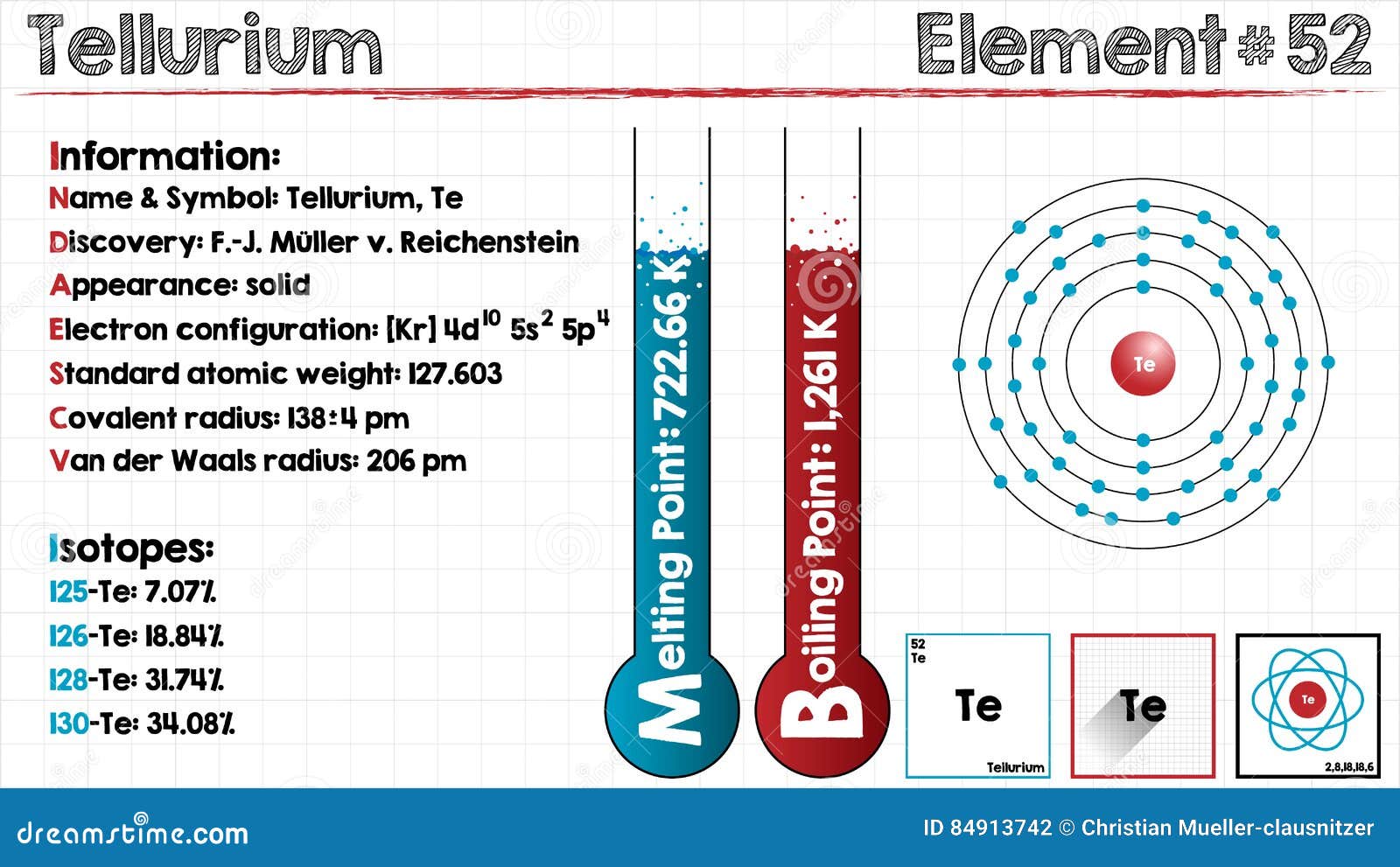
P Density g cm 3. In the case of Tellurium the abbreviated electron configuration is Kr 4d10 5s2 5p4. Noncombustible Solid in bulk form but will burn in powder form. This list contains the 118 elements of chemistry. It is occasionally found in native form as elemental crystals.
 Source: slideplayer.com
Source: slideplayer.com
In the case of Tellurium the abbreviated electron configuration is Kr 4d10 5s2 5p4. Tellurium trigonal Melting point of Tellurium trigonal 4498. Click on any elements name for further chemical properties environmental data or health effects. Melting point The temperature at which the solidliquid phase change occurs. Value given for yellow phosphorus form.
 Source: rsc.org
Source: rsc.org
5 - Inventor surname. In the case of Tellurium the abbreviated electron configuration is Kr 4d10 5s2 5p4. Melting Point of all the elements in the Periodic Table in Graph and Table format Complete information about all the properties of elements using Graphs and Tables Interactive Dynamic Periodic Table Periodic Table Element Comparison Element Property trends and complete information about the element - Facts How to Locate on Periodic Table History Abundance Physical Properties Thermal. Atomic number - Name alphabetically-272. Noncombustible Solid in bulk form but will burn in powder form.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. 865 metal Flash Point. At the melting point the solid and liquid phases exist in equilibrium. But melting and boiling points do. 56 - Year of discovery.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Symbols Melting Point Name 095 K-27205 C-458 F. 79 - Vanderwaals radius. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. The melting point is the highest temperature at which crystallization may occur. Boiling points increases on moving down from fluorine to iodine.
 Source: material-properties.org
Source: material-properties.org
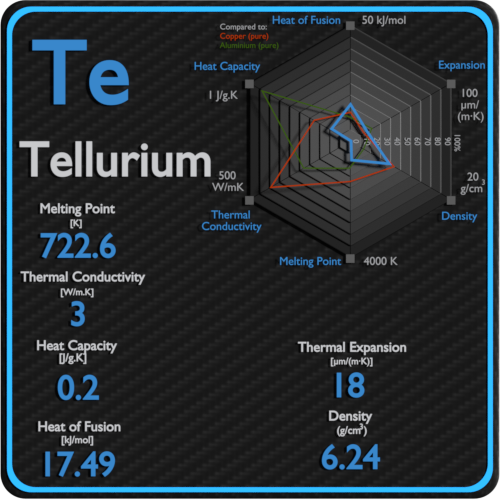
As the raw materials feeding the alloy continuously the Te in the SnTe alloy would be gradually saturated leading to the epitaxial growth of Te nanowires. The elements of the periodic table sorted by melting point. Up to date curated data provided by Mathematicas ElementData. -269 C -452 F. 4 - Elements in earthcrust.
 Source: lenntech.com
Source: lenntech.com
56 - Year of discovery. Symbols Melting Point Name 095 K-27205 C-458 F. 865 metal Flash Point. Value given for alpha form. It is also a temperature at which a solid crystal turns into a liquid.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of tellurium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.