Melting point of sodium iodide
Home » datasheet » Melting point of sodium iodideMelting point of sodium iodide
Melting Point Of Sodium Iodide. While the decahydrate sodium sulfate has been known as Glaubers salt or mirabilis. Anhydrous sodium sulfate is a white crystalline solid also known as the mineral thenardite. They are white crystals which do not have an odour but possess a taste. The physical properties of the chlorides of elements in Groups 1 and 2 are very different compared.
 Sodium Iodide Wikipedia From en.wikipedia.org
Sodium Iodide Wikipedia From en.wikipedia.org
Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O. Boiling Point C Feature. The physical properties of the chlorides of elements in Groups 1 and 2 are very different compared. Melting Point C Physical Form. Trends in melting and boiling points.
It is a strong base.
Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO It may also be viewed as the sodium salt of hypochlorous acidThe anhydrous compound is unstable and may decompose explosively. The figure above shows melting and boiling points of the Group 1 elements. First we must analyze compounds formed from elements from Groups 1 and 2 eg sodium and magnesium. Boiling Point C Feature. It has a melting point of 801C and a boiling point of. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O.
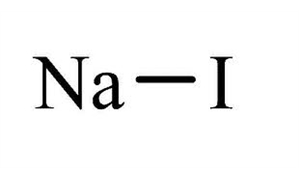 Source: lobachemie.com
Source: lobachemie.com
Lead iodide must be stored to avoid contact with oxidizers such as perchlorates peroxides permanganates chlorates and nitrates and chemically active metals such as potassium sodium magnesium and zinc since violent reactions occur. The melting point is correlated to the strength of intermolecular bonds within the element. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The octahydrate which is simple to prepare is white in contrast. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine.
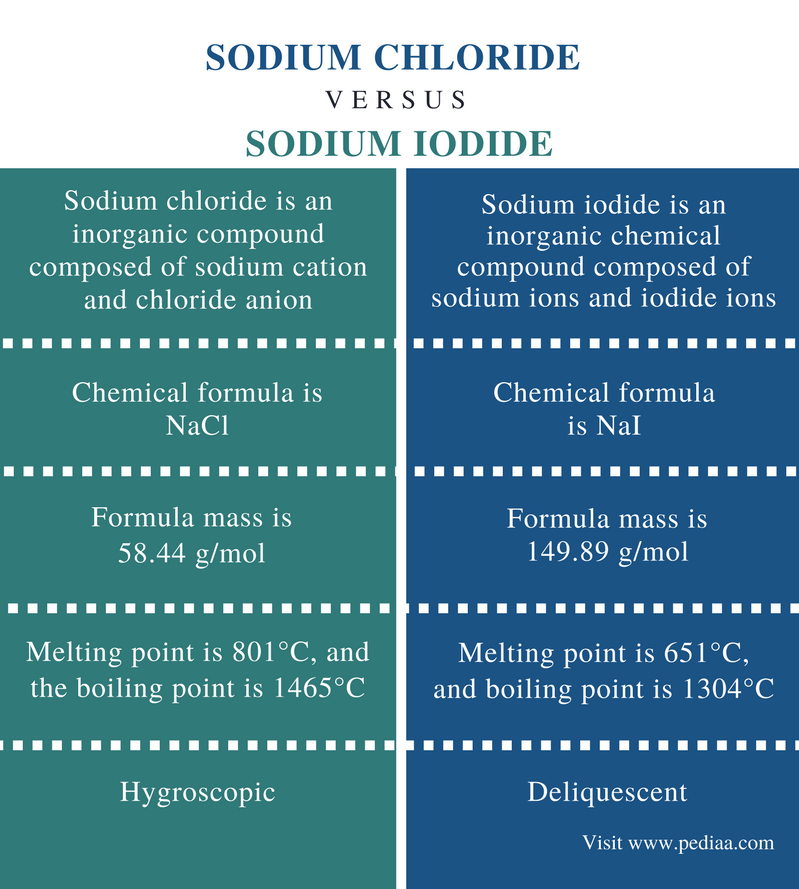 Source: pediaa.com
Source: pediaa.com
Both the melting and boiling points decrease down the group. It has a melting point of 801C and a boiling point of. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. It can be crystallized as a pentahydrate NaOCl 5 H. Sodium peroxide is the inorganic compound with the formula Na 2 O 2This yellowish solid is the product of sodium ignited in excess oxygen.
 Source: sodium.atomistry.com
Source: sodium.atomistry.com
Melting Point C Physical Form. When any of the Group 1 metals is melted the metallic bond is weakened enough for the atoms to move more freely and is broken completely when the boiling point is reached. While the decahydrate sodium sulfate has been known as Glaubers salt or mirabilis. It is easily soluble in water and partially soluble or insoluble in other liquids. Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO It may also be viewed as the sodium salt of hypochlorous acidThe anhydrous compound is unstable and may decompose explosively.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium peroxide is the inorganic compound with the formula Na 2 O 2This yellowish solid is the product of sodium ignited in excess oxygen. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. It has a melting point of 801C and a boiling point of. The melting point is correlated to the strength of intermolecular bonds within the element. First we must analyze compounds formed from elements from Groups 1 and 2 eg sodium and magnesium.
 Source: researchgate.net
Source: researchgate.net
It is easily soluble in water and partially soluble or insoluble in other liquids. In its aqueous state NaCl acts as a good conductor of electricity due to the free movement of the ions. It is a strong base. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. The figure above shows melting and boiling points of the Group 1 elements.
 Source: byjus.com
Source: byjus.com
It is a strong base. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. It is a strong base. It is easily soluble in water and partially soluble or insoluble in other liquids. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In its aqueous state NaCl acts as a good conductor of electricity due to the free movement of the ions. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O. Lead iodide must be stored to avoid contact with oxidizers such as perchlorates peroxides permanganates chlorates and nitrates and chemically active metals such as potassium sodium magnesium and zinc since violent reactions occur. It is easily soluble in water and partially soluble or insoluble in other liquids.
Source: chemspider.com
It is a strong base. It can be crystallized as a pentahydrate NaOCl 5 H. Lead iodide must be stored to avoid contact with oxidizers such as perchlorates peroxides permanganates chlorates and nitrates and chemically active metals such as potassium sodium magnesium and zinc since violent reactions occur. They are white crystals which do not have an odour but possess a taste. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine.
The melting point is correlated to the strength of intermolecular bonds within the element. The octahydrate which is simple to prepare is white in contrast. When any of the Group 1 metals is melted the metallic bond is weakened enough for the atoms to move more freely and is broken completely when the boiling point is reached. Boiling Point C Feature. Trends in melting and boiling points.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Lead iodide must be stored to avoid contact with oxidizers such as perchlorates peroxides permanganates chlorates and nitrates and chemically active metals such as potassium sodium magnesium and zinc since violent reactions occur. The octahydrate which is simple to prepare is white in contrast. Boiling Point C Feature. Properties of Sodium Chloride. Anhydrous sodium sulfate is a white crystalline solid also known as the mineral thenardite.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of sodium iodide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.