Melting point of potassium nitrate
Home » datasheet » Melting point of potassium nitrateMelting point of potassium nitrate
Melting Point Of Potassium Nitrate. Potassium is the seventh most abundant element found on Earths crust. After sodium chlorate it is the second most common chlorate in industrial use. Potassium Nitrate Chemical Formula. In theory the two temperatures would be the same but liquids can be supercooled beyond their freezing points so that they dont solidify until well below freezing point.
 The Heat Of Fusion Melting Temperature Of Kno3 Nano3 Solar Salt And Download Scientific Diagram From researchgate.net
The Heat Of Fusion Melting Temperature Of Kno3 Nano3 Solar Salt And Download Scientific Diagram From researchgate.net
Chemistry End of Chapter Exercises. It is also a temperature at which a solid crystal turns into a liquid. The melting point is specific for a given substance. Melting point is a characteristic property of solid crystalline substances. Melting point - the temperature at which a solid turns into a liquid. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials.
Potassium Nitrate Safety Data Sheet according to Federal.
SLP4009 SLP3001 SLP4816 SLP1987 CAS. The melting point is specific for a given substance. Potassium is the seventh most abundant element found on Earths crust. The alkali metals have low melting points ranging from a high of 179 C 354 F for lithium to a low of 285 C 833 F for cesium. The melting point depends on the pressure. If the temperature is below 57C for this mixture.
 Source: researchgate.net
Source: researchgate.net
After sodium chlorate it is the second most common chlorate in industrial use. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. In theory the two temperatures would be the same but liquids can be supercooled beyond their freezing points so that they dont solidify until well below freezing point. Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen. Carl Wilhelm Scheele a.
 Source: shutterstock.com
Source: shutterstock.com
The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. Not applicable Flash point. It is a strong oxidizing agent and its most important application is in safety matches. Chemical Product and Company Identification Product Name. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance.
 Source: youtube.com
Source: youtube.com
Additionally potassium metabisulfite is a disulfite and has a melting point of 374 degrees Fahrenheit. Carl Wilhelm Scheele a. You can think of this as a simple phase diagram. For example the melting point of ice frozen water is 0 C. Chemical Product and Company Identification Product Name.
 Source: chemicalbook.com
Source: chemicalbook.com
Used in the making of grass jelly. Potassium Carbonate Structure K 2 CO 3. Potassium nitrate also called saltpeter or niter a white solid soluble in water formed by fractional crystallization of sodium nitrate and potassium chloride solutions. Potassium is the seventh most abundant element found on Earths crust. K 2 CO 3 Uses Potassium carbonate Potassium carbonate is used as a mild drying agent.
 Source: researchgate.net
Source: researchgate.net
In theory the two temperatures would be the same but liquids can be supercooled beyond their freezing points so that they dont solidify until well below freezing point. If you have a mixture of 100 g of potassium nitrate and 100 g of water and the temperature is above 57C you have a single phase - a solution of potassium nitrate. Potassium nitrate also called saltpeter or niter a white solid soluble in water formed by fractional crystallization of sodium nitrate and potassium chloride solutions. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc. The aim of this study was to compare a 3 potassium nitrate02 sodium fluoride mouthwash with a 02 sodium fluoride control mouthwash in a 6-week double-blind study.

If the temperature is below 57C for this mixture. No data available Boiling point. The freezing point describes the liquid to solid transition while the melting point is the temperature at which water goes from a solid ice to liquid water. Potassium nitrate has been used. Potassium is the seventh most abundant element found on Earths crust.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Not applicable solid Relative evaporation rate butyl acetate1. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. It is used in research and development as well as in quality control in. It is also a temperature at which a solid crystal turns into a liquid. Potassium nitrate is used in gunpowder.
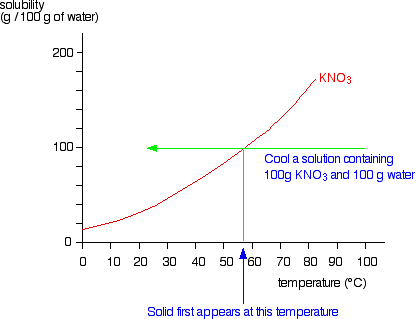 Source: chemguide.co.uk
Source: chemguide.co.uk
If exposed to the raw. The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. Melting point determination is the thermal analysis most frequently used to characterize solid crystalline materials. 334 C Freezing point. In October 1807 the English chemist Sir Humphry Davy isolated potassium and then sodium.
 Source: sciencenotes.org
Source: sciencenotes.org
About four-fifths of Earths atmosphere is nitrogen which was isolated and recognized as a specific substance during early investigations of the air. The melting point depends on the pressure. SLP4009 SLP3001 SLP4816 SLP1987 CAS. The alkali metals have low melting points ranging from a high of 179 C 354 F for lithium to a low of 285 C 833 F for cesium. Not applicable solid Relative evaporation rate butyl acetate1.
Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. 1s 2 2s 2 2p 3. Carl Wilhelm Scheele a. No data available Vapor pressure. The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of potassium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.