Melting point of p nitroaniline
Home » datasheet » Melting point of p nitroanilineMelting point of p nitroaniline
Melting Point Of P Nitroaniline. Add fuming nitric acid drop by drop carefully and do not inhale the fumes of nitric acid. Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. 3 2015 9 2.

The percutaneous absorption of nitrobenzene p-nitroaniline 24-dinitrochlorobenzene 2-nitro-p-phenylenediamine and 4-amino-2-nitrophenol was studied in vivo and in vitro. 2 2017 7 3. Add fuming nitric acid drop by drop carefully and do not inhale the fumes of nitric acid. Preparation of p-Nitroaniline This experiment usually takes three weeks to complete and counts as two laboratory experiments 40 points. Discontinuous multiphase polycrystalline alumina fibres in chopped fibre or random mat form containing 3 by weight or more silica with a specific modulus of less than 10 x 10 6 m. Five day urine samples were collected and analyzed for the compounds.
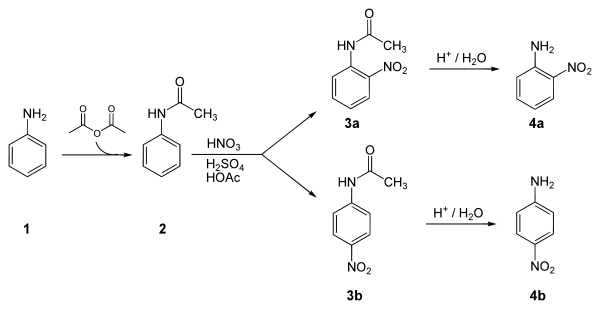
The overall reaction scheme is given in Figure 141 NH2 CH3 C O C CH3.
The percutaneous absorption of nitrobenzene p-nitroaniline 24-dinitrochlorobenzene 2-nitro-p-phenylenediamine and 4-amino-2-nitrophenol was studied in vivo and in vitro. Five day urine samples were collected and analyzed for the compounds. Synthesis References Journal of the American Chemical Society 75 p. The yield of p-Nitroacetanilide is _____gm. Synthetic human hair wigs are made from a copolymer of vinyl chloride and. Which has most stable 2 oxidation state.
 Source: tcichemicals.com
Source: tcichemicals.com
Hazardous Substances Data Bank HSDB 326 Solubility. Melting softening decomposition or sublimation point exceeding 1922 K 1649 C in an inert environment. In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements. 3 2015 9 2. Heat of fusion at melting point 236 kJmol.
 Source: scbt.com
Source: scbt.com
A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16. Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. Add fuming nitric acid drop by drop carefully and do not inhale the fumes of nitric acid. Does not apply to. A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16.

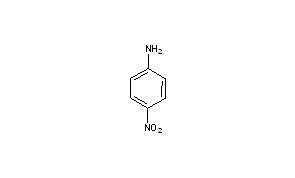
Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. 2 2016 8 19. 4-Nitroaniline p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C 6 H 6 N 2 O 2It is an organic chemical compound consisting of a benzene ring in which an amino group is para to a nitro groupThis chemical is commonly used as an intermediate in the synthesis of dyes antioxidants pharmaceuticals gasoline gum inhibitors poultry medicines and as a. D The requirements of this part shall not affect the availability of a waiver under section 121d4 of the Comprehensive Environmental Response Compensation and Liability Act of 1980 CERCLA. 2 2016 16 4.

3 2015 9 2. Does not apply to. Filter dry and record the melting point and TLC using toluene as solvent. How to recrystallize bong water. 1 2015 5 3.
 Source: prepchem.com
Source: prepchem.com
The yield of p-Nitroacetanilide is _____gm. E The following hazardous wastes are not subject to any. Your assessment is very important for improving the workof artificial intelligence which forms the content of this project. Discontinuous multiphase polycrystalline alumina fibres in chopped fibre or random mat form containing 3 by weight or more silica with a specific modulus of less than 10 x 10 6 m. Heat of fusion at melting point 236 kJmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The compounds were applied to shaved abdominal skins of Rhesus-monkeys at a concentration of 4 ugsq cm. Keep visiting BYJUS to learn more about. Add fuming nitric acid drop by drop carefully and do not inhale the fumes of nitric acid. We will do a three-step synthesis to make p- nitroaniline from aniline and then we will characterize our product using the new and very useful technique of thin layer chromatography TLC. E The following hazardous wastes are not subject to any.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16. 2 2016 16 6. 1 2015 5 3. Respirators used shall be. A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
CRC Handbook of Chemistry and Physics 86TH Edition 2005-2006. Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes. 1 2016 2 1. Temperature should not exceed more than 20 o C. The yield of p-Nitroacetanilide is _____gm.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Synthesis References Journal of the American Chemical Society 75 p. Index No International Chemical Identification EC No CAS No Classification Labelling Specific Conc. 3 2015 9 2. Preparation of p-Nitroaniline This experiment usually takes three weeks to complete and counts as two laboratory experiments 40 points. 2 2016 8 19.
 Source: drugfuture.com
Source: drugfuture.com
A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16. How to recrystallize bong water. 167 ugmL Burnham Center for Chemical Genomics. Less than 1 mgmL at 725 F NTP 1992 National Toxicology Program Institute of. In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of p nitroaniline by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.