Melting point of mgcl2
Home » datasheet » Melting point of mgcl2Melting point of mgcl2
Melting Point Of Mgcl2. When hit they produce sound. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. Product name Magnesium Chloride MgCl2 25mM Other means of identification Product No B9021 Recommended use of the chemical and restrictions on use Recommended use This product is for research and development only Restrictions on use No information available Details of the supplier of the safety data sheet Company Phone Number 978-927-5054 800-632-5227 toll free Telefax 978-921. Which of the following solids would have the highest melting point.
 Magnesium Chloride Wikipedia From en.wikipedia.org
Magnesium Chloride Wikipedia From en.wikipedia.org
Recommended use and restrictions on use Use of the substancemixture. Melting LightCycler 480 High Resolution Melting Master 04 909 631 001 5 mL 5 x 1 mL 2x concentrated 25 mL MgCl2 stock solution for 500 20 µL reactions Hybridization Probes LightCycler 480 Genotyping Master 04 707 524 001 15 mL 5x concentrated Master Mix for 250 20 µL reactions Hydrolysis Probes TaqMan Probes or Universal Probe Library UPL LightCycler 480 Probes Master. Before this point hydration only happened in the cavity. Sodium and chloride ions represent in 11 in NaCl chemical formula. AlCl3 NaCl MgCl2 d. Hg and Br are liquid H2 N2 O2 F2 Cl2 and the inert gases are.
A NaF B NaCl C NaBr D NaI.
Over time one becomes familiar with certain substances. An ion and a water molecule. Na O2 Na2O. Which of the following solids would have the highest melting point. If the experiment is repeated using solutions of the same molarity which of the following changes in volume will double the amount of MgOH2s produced. NaCl AlCl3 MgCl2 9.
 Source: researchgate.net
Source: researchgate.net
If individual is experiencing respiratory discomfort or irritation remove to fresh air. Alkali metals lithium sodium potassium are so soft that they can be cut with a knife. If we notice the nature of oxides. Which of the following solids would have the highest melting point. Ii K is larger than Na so NaCl has a higher lattice energy and a higher melting point than KCl.
 Source: chem-solutions-9701.blogspot.com
Source: chem-solutions-9701.blogspot.com
This phenomenon is more extensively discussed in Section 24. A KI B KBr C KCl D KF. Over time one becomes familiar with certain substances. Ingesting MgCl2 brine will usually cause purging of the stomach by vomiting. Q-Solution an innovative PCR additive that facilitates amplification of difficult templates by modifying the melting behavior of DNA is also provided with HotStarTaq DNA Polymerase.
 Source: oneclass.com
Source: oneclass.com
Sodium and chloride ions represent in 11 in NaCl chemical formula. Platinum is an allotrope of carbon is the hardest natural substance known and has a very. The rest are liquids or low melting point solids. Metal oxygen metal oxide. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great.

Technically the lattice energy. Metals high mpt and bpt Non metals. AlCl3 MgCl2 NaCl e. Recommended use and restrictions on use Use of the substancemixture. A KI B KBr C KCl D KF.
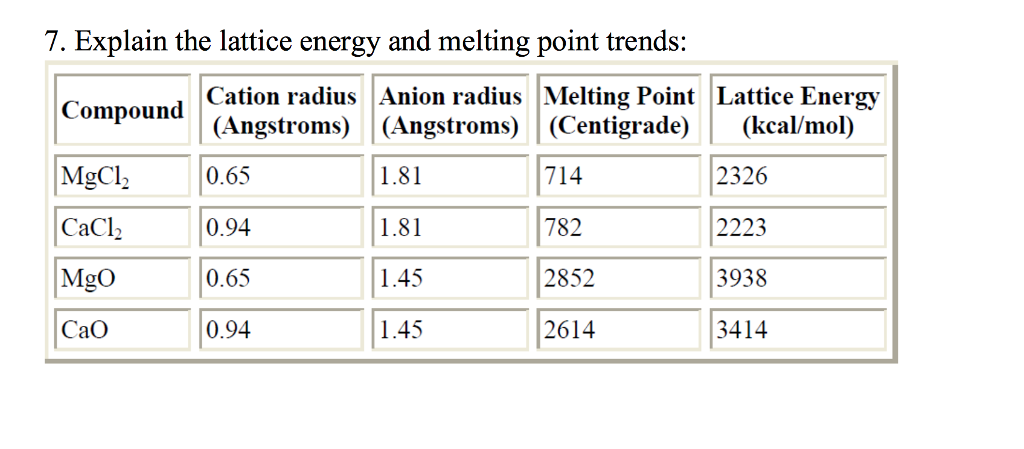 Source: chegg.com
Source: chegg.com
Solutions of Class 10 Science Sample Paper Term-1 2021-22 CBSE Board Solutions of Class 10 Science Sample Paper Term-1 2021-22 CBSE Board is helpful for the class 10 students in their preparation of the term 1 CBSE board exam 2021. Aside from looking at. The formula H2O is also the molecular formula of water. Mg2 Na H-Br N 2 10. Before this point hydration only happened in the cavity.
 Source: kolibri.teacherinabox.org.au
Source: kolibri.teacherinabox.org.au
NaCl AlCl3 MgCl2 9. Technically the lattice energy. Using the same volume of. Which of the following solids would have the highest melting point. If discomfort or irritation persists get medical attentionadvice.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Product name Magnesium Chloride MgCl2 25mM Other means of identification Product No B9021 Recommended use of the chemical and restrictions on use Recommended use This product is for research and development only Restrictions on use No information available Details of the supplier of the safety data sheet Company Phone Number 978-927-5054 800-632-5227 toll free Telefax 978-921. Please use this book to increase your knowledge for the laboratory pratictioner. Laboratory chemicals Restrictions on use. This formula implies that the water molecules consist of 2 hydrogen and 1 oxygen atoms. 17944 19659 Initial pressure kPa 100.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1 Reaction With Air. If individual is experiencing respiratory discomfort or irritation remove to fresh air. A NaI B NaF C MgO D MgCl2 E KF. Product name Magnesium Chloride MgCl2 25mM Other means of identification Product No B9021 Recommended use of the chemical and restrictions on use Recommended use This product is for research and development only Restrictions on use No information available Details of the supplier of the safety data sheet Company Phone Number 978-927-5054 800-632-5227 toll free Telefax 978-921. NaCl NaBr NaI Melting point K 1074 1020 920 Suggest why the melting point of sodium iodide is lower than the melting point of sodium bromide.
 Source: scbt.com
Source: scbt.com
This phenomenon is more extensively discussed in Section 24. All metals except mercury exist as solids at room temperature. Metalssonorous Non metalnon sonorous. Hg and Br are liquid H2 N2 O2 F2 Cl2 and the inert gases are. Over time one becomes familiar with certain substances.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This phenomenon is more extensively discussed in Section 24. Magnesium Chloride MgCl2 - Magnesium Chloride is the chemical name of MgCl2. Often a concrete-safe and pet-safe ice melt option. Shop Ice Melt Shop Spreaders Sprayers Shop Spray-On Windshield De-Icers. Over time one becomes familiar with certain substances.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of mgcl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.