Melting point of hydrogen fluoride
Home » datasheet » Melting point of hydrogen fluorideMelting point of hydrogen fluoride
Melting Point Of Hydrogen Fluoride. Melting Point MP Hydrogen changes its state from solid to liquid at -252762C -4229716F or 20388K Boiling Point BP Hydrogen changes its state from liquid to gas at -25916C -434488F or 1399K Hydrogen is a colorless odorless gas. Substance Formula Melting point C Boiling temperature C Density 25C. Hydrogen fluoride mixes readily with water forming hydrofluoric acid. For all practical purposes they are considered the same chemical.
Out Of Hf Hcl Hbr And Hi Which Has The Lowest And Highest Boiling Point And Why Quora From quora.com
We would like to show you a description here but the site wont allow us. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. This agent may also inhibit acid production by commensal oral bacteria. H 2 TeO 3. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid.
Physical Properties of Sodium fluoride NaF.
Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. This agent may also inhibit acid production by commensal oral bacteria. Sodium Fluoride Structure NaF. Substance Formula Melting point C Boiling temperature C Density 25C. 10 to 50 mgmL at 73 F. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
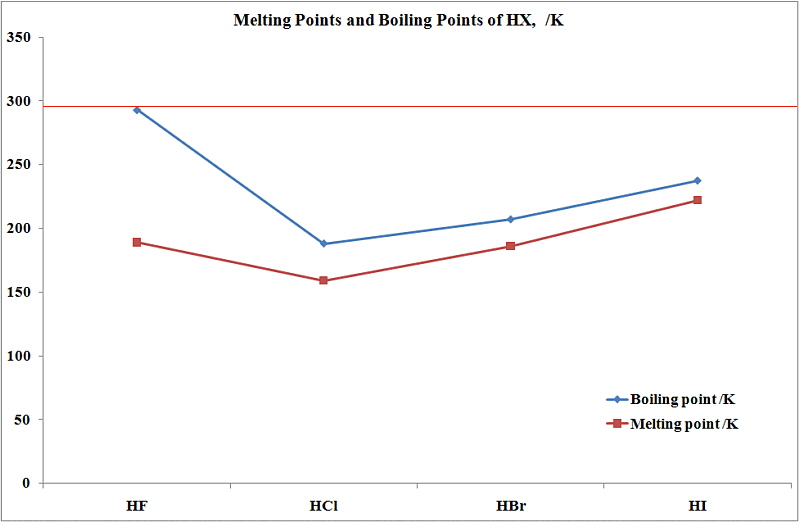 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
May cause irritation of the digestive tract and possible burns. This white salt is hygroscopic and even deliquescentSamples should therefore be protected from sources of moisture including the water vapor present in ambient air. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. Sodium Fluoride Structure NaF. We would like to show you a description here but the site wont allow us.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Chemical Properties of Sodium fluoride NaF. Exposure to fluoride compounds can result in systemic toxic effects on the heart liver and kidneys. Hydrogen fluoride mixes readily with water forming hydrofluoric acid. H 2 TeO 3. Sodium Fluoride Structure NaF.
Source: chemguide.co.uk
This colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acid. H 3 PO 4. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. The strong hydrogen bonds involve ionic species. Chemical Properties of Sodium fluoride NaF.
 Source: youtube.com
Source: youtube.com
Examples include Cl H 2 O F H 2 O H 3 O H 2 O and F HF with interaction energies of. Examples include Cl H 2 O F H 2 O H 3 O H 2 O and F HF with interaction energies of. It is easily ignited and once ignited it burns with a pale blue almost invisible flame. It is an intermediate for many chemical reactions and syntheses. We would like to show you a description here but the site wont allow us.
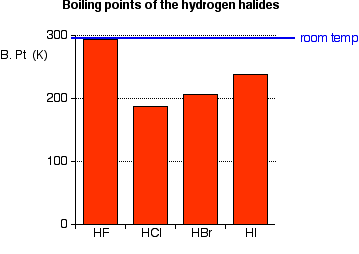 Source: chemguide.co.uk
Source: chemguide.co.uk
Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates. This agent may also inhibit acid production by commensal oral bacteria. Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates. It may also deplete calcium levels in the body leading to hypocalcemia and death. Sodium fluoride reacts with water.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Melting Point MP Hydrogen changes its state from solid to liquid at -252762C -4229716F or 20388K Boiling Point BP Hydrogen changes its state from liquid to gas at -25916C -434488F or 1399K Hydrogen is a colorless odorless gas. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. 10 to 50 mgmL at 73 F. White crystals or powder. HF is widely used in the petrochemical.
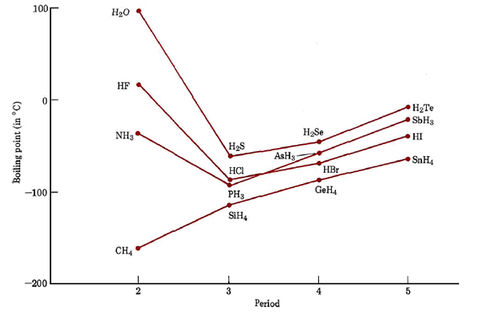 Source: chem.libretexts.org
Source: chem.libretexts.org
Melting point C. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. May cause respiratory. Other well-known dimers in this group involve carboxylic acids base pairs of nucleic acids and typical hydrogen bonds forming within or between proteins.
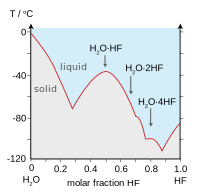 Source: en.wikipedia.org
Source: en.wikipedia.org
Water dimer or hydrogen fluoride dimer are typical examples for this group. For all practical purposes they are considered the same chemical. This colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acid. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries.
Source: quora.com
1 mm Hg at 1971 F. The hydrogen vapors are lighter than air. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. Melting Point MP Hydrogen changes its state from solid to liquid at -252762C -4229716F or 20388K Boiling Point BP Hydrogen changes its state from liquid to gas at -25916C -434488F or 1399K Hydrogen is a colorless odorless gas. 1 mm Hg at 1971 F.
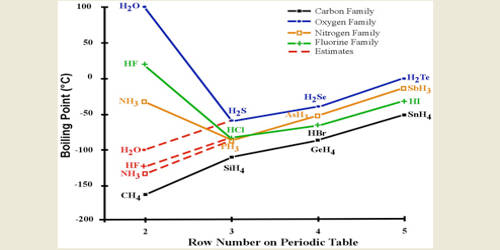 Source: qsstudy.com
Source: qsstudy.com
It is used. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. Chemical Properties of Sodium fluoride NaF. Hydrogen fluoride is a chemical compound with the chemical formula H F. Melting Point MP Hydrogen changes its state from solid to liquid at -252762C -4229716F or 20388K Boiling Point BP Hydrogen changes its state from liquid to gas at -25916C -434488F or 1399K Hydrogen is a colorless odorless gas.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of hydrogen fluoride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.