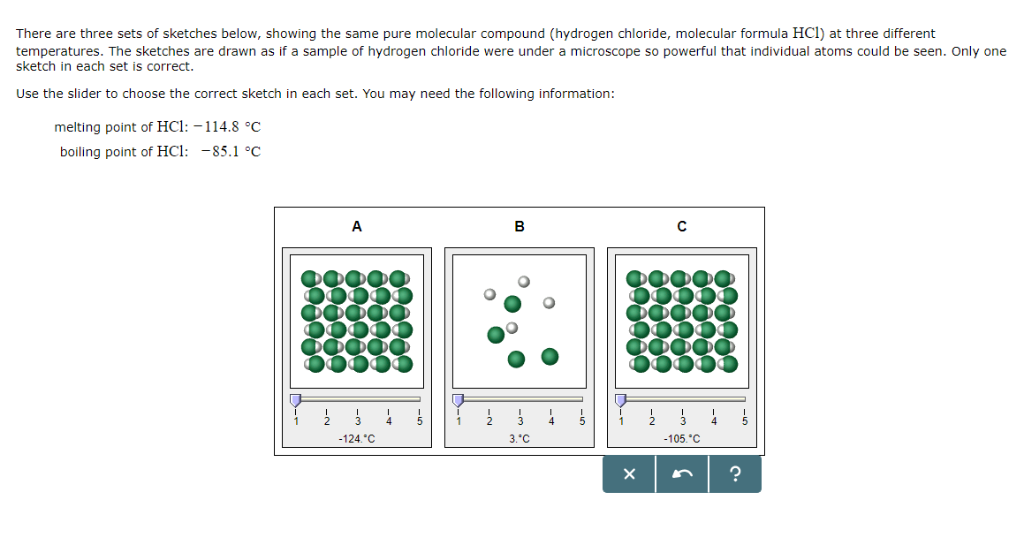
Melting point of hcl
Home » datasheet » Melting point of hclMelting point of hcl
Melting Point Of Hcl. Equilibrium melting point of quartz is 1720 C with a flux-adjusted melting point of 1520 C. Why is the PH of a base bigger than 7SecondAt the equivalence point of a titration between acetic acid weak acid and sodium hydroxide strong base. Experimental Melting Point-1142 C OU Chemical Safety Data No. Branched —more sphere-like - - lower surface area — lower boiling point.
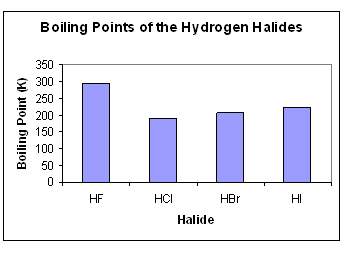 Question 94828 Socratic From socratic.org
Question 94828 Socratic From socratic.org
Prevents Infections Hydrochloric acid acts as a barrier against foreign microorganisms and helps prevent infection. H302 H315 H319 H335 H410. So here are the key relationships between branching and meltingboiling points. Samantha Bidwell Created Date. A from level 457 sample E313 413 cm depth. The melting point was measured using the Büchi B-450 device by the capillary technique 51.
83 deg C 760 mmHg FreezingMelting Point-66 deg C Decomposition TemperatureNot available.
Q4 Give an explanation in terms of intermolecular forces for the following differences in boiling point. T FP -k f m. Phase at room temperature. Therefore you should obtain the melting point and weight of this compound before leaving the lab. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Preparation of Hydrochloric acid HCl.
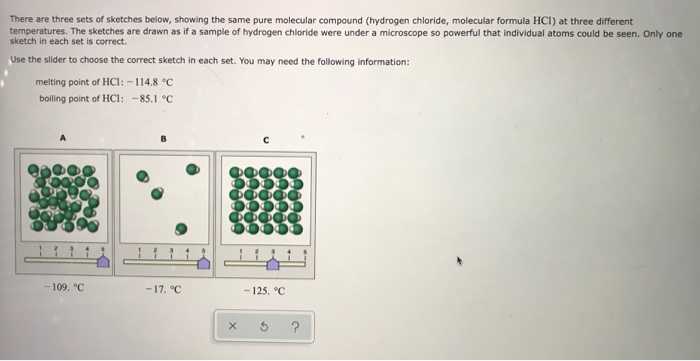 Source: chegg.com
Source: chegg.com
After sodium chlorate it is the second most common chlorate in industrial use. Preparation of Hydrochloric acid HCl. 383 C 721 F. Q4 Give an explanation in terms of intermolecular forces for the following differences in boiling point. They are easily melted in a boiling tube placed in a beaker of hot water.
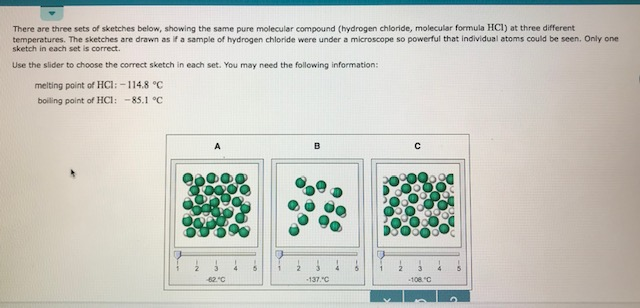 Source: chegg.com
Source: chegg.com
This is because CH3COOH reacts with OH- produced water and CH3OO- which is a relatively strong conjugate base. The PH is around 9. The melting point depends on the pressure. So here are the key relationships between branching and meltingboiling points. We say that such a body melts.

Hydrochloric acid HCl aq is a strong acid meaning that when it is dissolved in water all the molecules ionize to form hydrogen ions Haq. 383 C 721 F. Chemical Properties of Hydrochloric acid. Linear versus branched — higher meltingboiling points due to better stacking and surface area contact. Top it up to the mark.
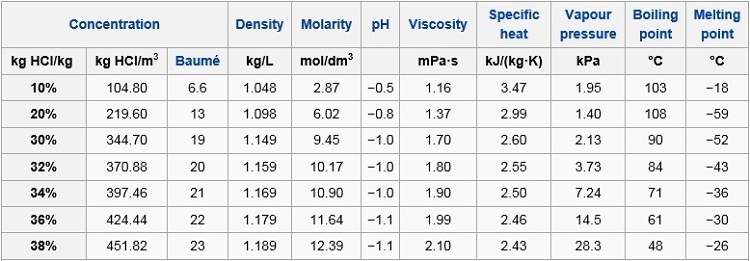 Source: hydro-land.com
Source: hydro-land.com
A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. 656 K sublimes Solubility in water. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. Phase at room temperature. CH3OO- react with water produce some OH- thus increase the PH at the equivalence point.
 Source: socratic.org
Source: socratic.org
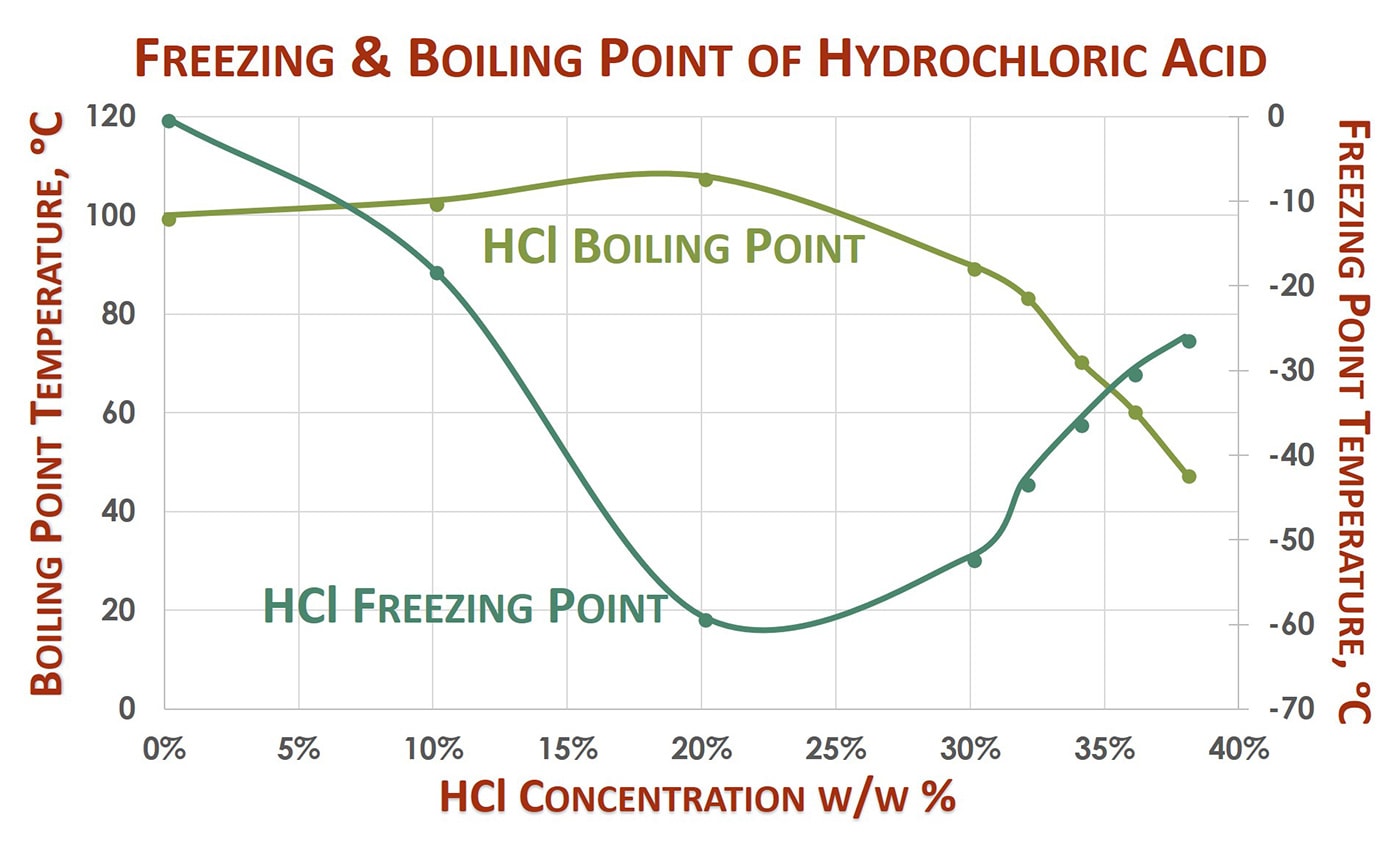
The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Here are some important functions of hydrochloric acid HCL in the stomach. Next week you will obtain the weights and melting points of benzoic acid and 4-chloroaniline and you can re-take the melting point naphthalene if any is left. The liquid may be cooled by putting the boiling tube in a beaker of cold water or just leaving it in the air.
 Source: chegg.com
Source: chegg.com
Therefore you should obtain the melting point and weight of this compound before leaving the lab. 383 C 721 F. Research Triangle Park North Carolina. Charlier GO Gas chromatographic separation of hydrogen chloride hydrogen sulfide and water Anal. They are easily melted in a boiling tube placed in a beaker of hot water.

Magnetic susceptibility χ 26010 6 cm 3 mol Refractive index n D 1973 Hazards Safety data sheet. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. After sodium chlorate it is the second most common chlorate in industrial use. Insoluble in ethanol ether. Chemical Properties of Hydrochloric acid.

Q2 To go. 02 mg100 mL Solubility product K sp 143 10 18. Therefore you should obtain the melting point and weight of this compound before leaving the lab. For example the melting point of ice frozen water is 0 C. This vitamin is essential to red blood cell nervous system and immune systems.
 Source: youtube.com
Source: youtube.com
Chem 393 1967 396-397 NIST Spectra nist ri. We say that such a body melts. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. This is because CH3COOH reacts with OH- produced water and CH3OO- which is a relatively strong conjugate base. A from level 457 sample E313 413 cm depth.
 Source: protank.com
Source: protank.com
Equilibrium melting point of quartz is 1720 C with a flux-adjusted melting point of 1520 C. Branched —more sphere-like - - lower surface area — lower boiling point. So here are the key relationships between branching and meltingboiling points. Next week you will obtain the weights and melting points of benzoic acid and 4-chloroaniline and you can re-take the melting point naphthalene if any is left. Melting point of ImCl was measured 1373 C which confirmed that ImCl is a solid salt and it is not an IL a salt in the liquid state with melting point lower than 100 C like most of alkyl imidazolium halides.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.