Melting point of hbr
Home » datasheet » Melting point of hbrMelting point of hbr
Melting Point Of Hbr. GHG emissions are the ideal starting point for such an approach. 14 mw86 has a boiling point of 68º. 59 C 138 F specific gravity. The launch of Pokémon Go this summer was a huge successboth for the gaming industry and for Augmented Reality AR.
 Hydrogen Halides As Acids From chemguide.co.uk
Hydrogen Halides As Acids From chemguide.co.uk
Mark each of the following statements as TRUE or FALSE. Substance Formula Melting point C Boiling temperature C Density 25C. The launch of Pokémon Go this summer was a huge successboth for the gaming industry and for Augmented Reality AR. Properties of Liquids. The high melting and boiling point of the compound. Why do compounds having hydrogen bonding have high melting and boiling points.
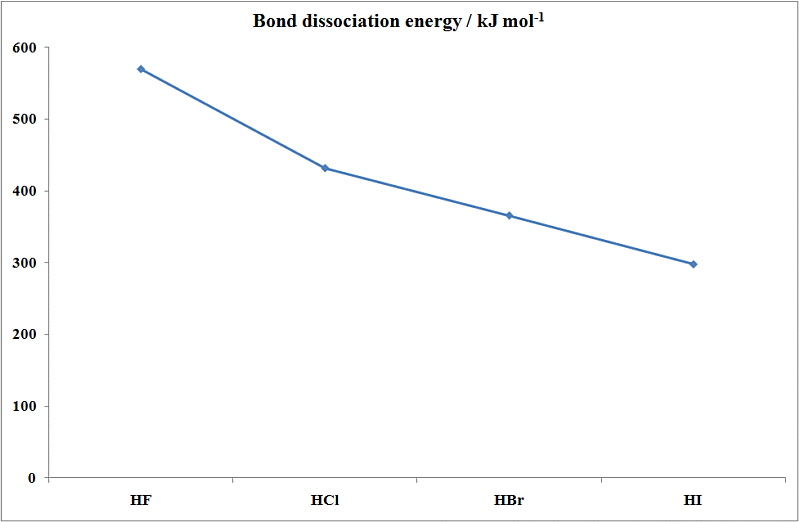
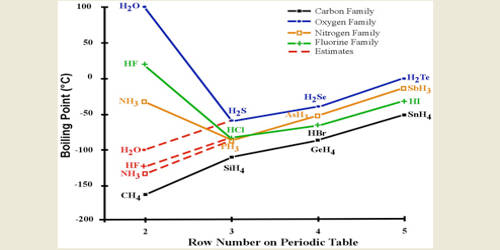
On the other hand there is a regular decrease in the first ionization energy as we go down this column.
312 at 20 C 68 F oxidation states. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. Ethanol has a higher boiling point because of greater London dispersion force c. Its melting point is -23C. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. This is due to hydrogen bonding in HF.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Ethanol has a higher boiling point because of greater London dispersion force c. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. Also relative molecular mass 1 is very low. The launch of Pokémon Go this summer was a huge successboth for the gaming industry and for Augmented Reality AR. This is due to hydrogen bonding in HF.
 Source: chemguide.co.uk
Source: chemguide.co.uk
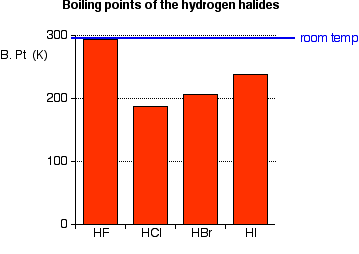
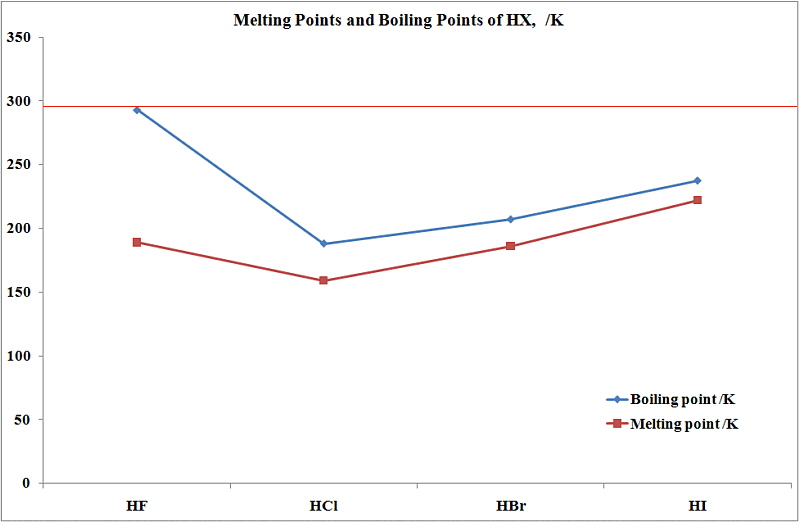
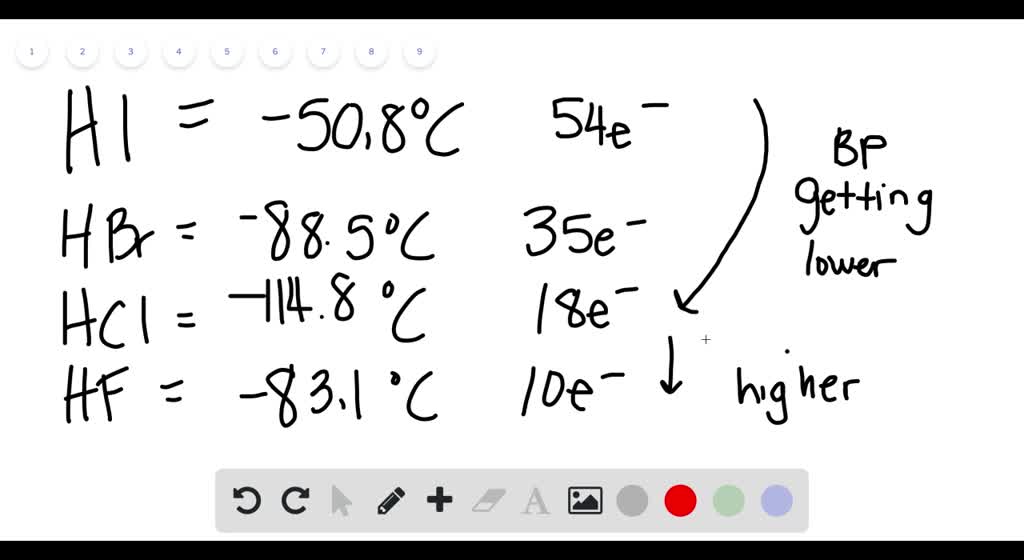
HBr has 36e-and HCl only 18 e-. However its bp189K is lower than that of the less polar HBr 206 K. The compounds having hydrogen bonding show abnormally high melting and boiling points. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. Properties of Liquids.

312 at 20 C 68 F oxidation states. He liberated the element by passing chlorine through. HBr has 36e-and HCl only 18 e-. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues from the manufacture of sea salt at Montpellier. When the temperature reaches 0 o C the melting point of ice further addition of heat does not change the temperature.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Ethanol must have stronger intermolecular attraction based on its higher boiling point. This is a list showing the boiling points and melting points of saturated and unsaturated hydrocarbons with same number of carbons. Substance Formula Melting point C Boiling temperature C Density 25C. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. However its bp189K is lower than that of the less polar HBr 206 K.
 Source: numerade.com
Source: numerade.com
This is due to the LDF. We say that such a body melts. From McGraw-Hill Dictionary of Scientific and Technical Terms 4th ed. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice. The melting point is the highest temperature at which crystallization may occur.

Electron configuration Ar3d 10 4s 2 4p 5. Hydrogen bromide is the inorganic compound with the formula H BrIt is a hydrogen halide consisting of hydrogen and bromine. For example the molecule carbon tetrachloride is a non-polar covalent molecule CCl 4. Molecular size is important but shape is also. Its melting point is -23C.
 Source: qsstudy.com
Source: qsstudy.com
We say that such a body melts. After launching in July. GHG emissions are the ideal starting point for such an approach. Properties of Liquids. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice.
 Source: chegg.com
Source: chegg.com
Water will bead-up on a waxed surface. Note that benzene is a liquid in room temperature and pressure. Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues from the manufacture of sea salt at Montpellier. Ethanol has a higher boiling point because of greater London dispersion force c. Number of carbon MeltingBoiling point Alkane Alkene Alkyne 2 Melting point ethane-183 ethene-169 ethyne-807 Boiling point ethane-89 ethene-104 ethyne.

Helium He is the element which has lowest melting point -2722 0 C. Which element has the lowest melting point element in periodic table. GHG emissions are the ideal starting point for such an approach. This is a list showing the boiling points and melting points of saturated and unsaturated hydrocarbons with same number of carbons. He liberated the element by passing chlorine through.
 Source: chegg.com
Source: chegg.com
72 C 19 F boiling point. 2 H 6 CH 3 NH 2 KCl CH 3 CH 2 CH 2 OH CH 3 OCH 3. Helium He is the element which has lowest melting point -2722 0 C. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. Substance Formula Melting point C Boiling temperature C Density 25C.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of hbr by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.