Melting point of fluorene
Home » datasheet » Melting point of fluoreneMelting point of fluorene
Melting Point Of Fluorene. Fluorene ˈ f l ʊər iː n or 9H-fluorene is an organic compound with the formula C 6 H 4 2 CH 2It forms white crystals that exhibit a characteristic aromatic odor similar to that of naphthaleneIt has a violet fluorescence hence its nameFor commercial purposes it is obtained from coal tar. Table 11 Melting Point Knowns Compound Literature mp C A biphenyl 69-72 25-dimethyl phenol 68-71 B 1-chloro-4-nitrobenzene 83. The temperature of the melting point dropped especially under reducing atmosphere as ashing temperature dropped and the potassium compounds increased. 153-154 C Oakwood 008846.
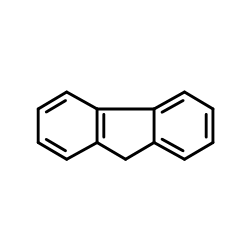 9h Fluorene Cas 86 73 7 Chemsrc From chemsrc.com
9h Fluorene Cas 86 73 7 Chemsrc From chemsrc.com
155-157 C SynQuest 2702-1-05. Melting points were measured by differential scanning calorimetry DSC using a NETZSCH DSC 204 F1 Phoenix instrument at a heating rate of 10 K min 1 under the protection of nitrogen. It is insoluble in water and soluble in many organic solvents. 155-157 C Cayman Chemical old CM250204. Furthermore the catalytic properties of copper are used for size-dependent dye. To help you understand the chemical basis of this exercise you should review Sections 35 - 37 in Solomons Fryhl which concerns the properties of acids and their conjugate bases.
You should also review the appropriate pages in the Mohrig.
MeltingBoiling point Alkane Alkene Alkyne 2 Melting point ethane-183 ethene-169 ethyne-807 Boiling point ethane-89 ethene-104 ethyne-847 3 Melting point propane-190 propene-185 propyne-1027 Boiling point propane-42 propene-47 propyne-232 4 Melting point butane-138 1-butene-1853 1-butyne-1257 Boiling point butane-05 1-butene-62. 156 C Jean-Claude Bradley Open Melting Point Dataset 6291. Furthermore the catalytic properties of copper are used for size-dependent dye. To help you understand the chemical basis of this exercise you should review Sections 35 - 37 in Solomons Fryhl which concerns the properties of acids and their conjugate bases. Fluorene ˈ f l ʊər iː n or 9H-fluorene is an organic compound with the formula C 6 H 4 2 CH 2It forms white crystals that exhibit a characteristic aromatic odor similar to that of naphthaleneIt has a violet fluorescence hence its nameFor commercial purposes it is obtained from coal tar. An additive-assisted polyol approach allows the synthesis of copper colloids of distinct sizes and properties which is an otherwise challenging task.
 Source: chemsrc.com
Source: chemsrc.com
Organic Chemistry vol 2 - IL. FILTER SORBENT 2-µm 37-mm PTFE washed XAD. The resulting polymers show liquid crystalline behavior good solubilities and molecular weight distributions. 155-157 C Cayman Chemical old CM250204. Other PAHs may be contained in asphalt used for the construction of roads in addition to roofing tar.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Furthermore the polymers display a blue emission. High efficiency and low efficiency roll off in white phosphorescent organic lightemitting diodes by managing host. FILTER SORBENT 2-µm 37-mm PTFE washed XAD. Table 11 Melting Point Knowns Compound Literature mp C A biphenyl 69-72 25-dimethyl phenol 68-71 B 1-chloro-4-nitrobenzene 83. Furthermore the polymers display a blue emission.
 Source: chemsynthesis.com
Source: chemsynthesis.com
It is insoluble in water and soluble in many organic solvents. It has been suggested that such fluorene-based click polymers are promising materials for applications in. 155-157 C Cayman Chemical old CM250204. The PVC bathing shoes were found to have 546 mgkg of 16 PAHs among all fluorene and phenanthrene levels were highest 170 and 120 mgkg respectively. Furthermore specific refined products of precise PAHs.
 Source: scielo.br
Source: scielo.br
CRC Handbook of Chemistry and Physics. Santos et al J. High efficiency and low efficiency roll off in white phosphorescent organic lightemitting diodes by managing host. It is insoluble in water and soluble in many organic solvents. 154 C Biosynth Q-101164.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Manufacture of resins and pesticides. Besides reading the lab manual you will need to do a little bit more. 155-157 C SynQuest 2702-1-05. White Powder Sublimation is a technique used to obtain ultra pure-grade chemicals. The PVC bathing shoes were found to have 546 mgkg of 16 PAHs among all fluorene and phenanthrene levels were highest 170 and 120 mgkg respectively.

TLC helps one determine the number of compounds in a mixture. 155-157 C Alfa Aesar L01917. It is insoluble in water and soluble in many organic solvents. INTRODUCTION Thin Layer Chromatography is an analytical technique that is simple and inexpensive. To help you understand the chemical basis of this exercise you should review Sections 35 - 37 in Solomons Fryhl which concerns the properties of acids and their conjugate bases.

The resulting polymers show liquid crystalline behavior good solubilities and molecular weight distributions. Water Organic solvents 150200 parts Miscible with alcohol ether fixed or volatile oils Merck 1989 Merck 1989. Manufacture of pharmaceuticals pigments dyes pesticides and thermoset plastic. 153-154 C Sigma-Aldrich ALDRICH-H31204. The temperature of the melting point dropped especially under reducing atmosphere as ashing temperature dropped and the potassium compounds increased.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Furthermore the polymers display a blue emission. Melting point No data Boiling point 203 EC Merck 1989 Specific gravity at 25 EC 108 Merck 1989 Odor Characteristic smokey odor Merck 1989 Taste Caustic burning taste Merck 1989 Odor threshold. An additive-assisted polyol approach allows the synthesis of copper colloids of distinct sizes and properties which is an otherwise challenging task. INTRODUCTION Thin Layer Chromatography is an analytical technique that is simple and inexpensive. It is insoluble in water and soluble in many organic solvents.
155-157 C Alfa Aesar L01917. Melting point 272 - 277 C lit Colour. Furthermore specific refined products of precise PAHs. 155-157 C Chemenu CM250204. To help you understand the chemical basis of this exercise you should review Sections 35 - 37 in Solomons Fryhl which concerns the properties of acids and their conjugate bases.
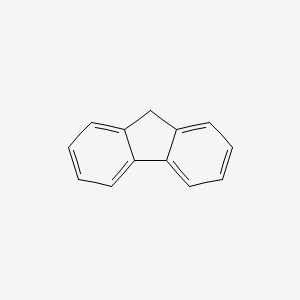
 Source: en.wikipedia.org
Source: en.wikipedia.org
Besides reading the lab manual you will need to do a little bit more. Melting point No data Boiling point 203 EC Merck 1989 Specific gravity at 25 EC 108 Merck 1989 Odor Characteristic smokey odor Merck 1989 Taste Caustic burning taste Merck 1989 Odor threshold. Table 11 Melting Point Knowns Compound Literature mp C A biphenyl 69-72 25-dimethyl phenol 68-71 B 1-chloro-4-nitrobenzene 83. Fluorene co-polymers with high efficiency deep blue electroluminescence J. 155-157 C Cayman Chemical old CM250204.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of fluorene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.