Melting point of flourine
Home » datasheet » Melting point of flourineMelting point of flourine
Melting Point Of Flourine. Helium does not solidify at standard pressure. Energy of third ionisation. Hydrogen has a melting point of -25914 C and a boiling point of -25287 C. In general boiling is a phase change of a substance from the liquid to the gas phase.
 G A C H P1 S1 Q1 A Elevise From elevise.co.uk
G A C H P1 S1 Q1 A Elevise From elevise.co.uk
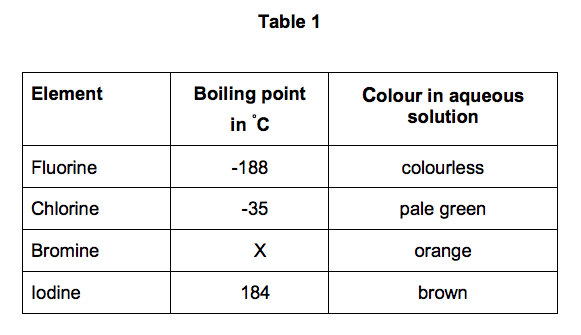
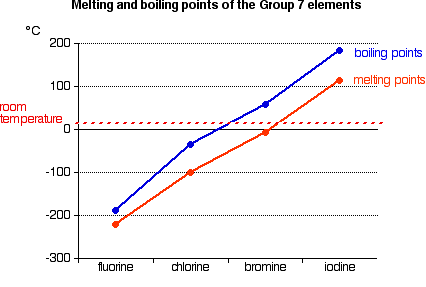
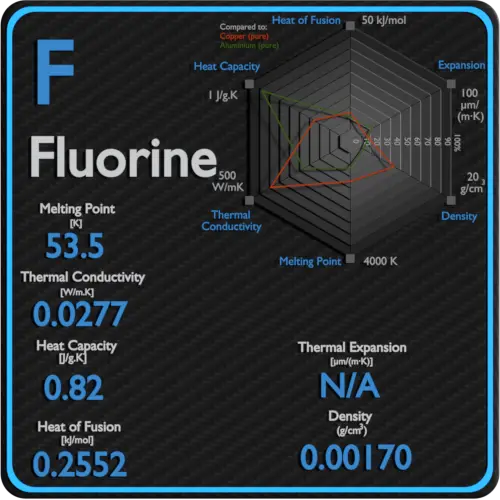
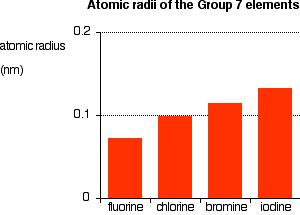
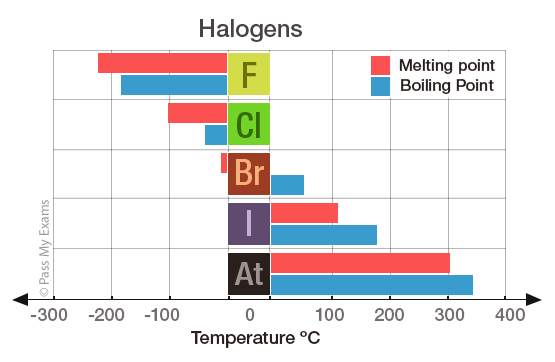
A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. At the melting point the solid and liquid phases exist in equilibrium. At normal atmospheric pressure carbon does not melt when heated it sublimes. Among the elements fluorine ranks 24th in universal abundance and 13th in. For this reason fluorine does not occur free in nature and was extremely difficult for scientists to isolate. Melting point of Fluorine is -2198C.
This list contains the 118 elements of chemistry.
Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. Electronic shell He 2s 2 2p 5. Boiling point of Fluorine is -1881C. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Melting point C. For this reason fluorine does not occur free in nature and was extremely difficult for scientists to isolate.
 Source: chemguide.co.uk
Source: chemguide.co.uk
If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. Among the elements fluorine ranks 24th in universal abundance and 13th in. Boiling point The temperature at which the liquidgas phase change occurs. Fluorine Melting Point and Boiling Point. 24867 C 4155 F boiling point.
 Source: slideplayer.com
Source: slideplayer.com
Value given for hexagonal gray form. Value given for monoclinic beta form. Melting point of Fluorine is -2198C. Fluorine can form ionic bonds with some elements such as carbon and boron and neon does not tend to form any bonds at all. This means that it will be solid at room temperature.
 Source: sites.google.com
Source: sites.google.com
Standard potential - 287 V. Iron Heavy Metal. C 4 H 8-1853. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. C 3 H 4-1027.
 Source: material-properties.org
Source: material-properties.org
Classify the six underlined properties in the following paragraph as chemical or physical. Adding a heat will convert the solid into a liquid with no temperature change. Melting point C. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Let us look at the elements in the ascending order of their melting points.
 Source: lizzyfluorine.weebly.com
Source: lizzyfluorine.weebly.com
A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. The halogens are five non-metallic elements found in group 17 of the periodic table. Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Hydrogen exists in two different spin isomers of hydrogen diatomic molecules that differ by the.
 Source: chemguide.co.uk
Source: chemguide.co.uk
At normal atmospheric pressure arsenic does not melt when heated. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. Hydrogen has a melting point of -25914 C and a boiling point of -25287 C. Up to date curated data provided by Mathematicas ElementData. Pure iron Fe has a fixed melting point of 1535 C chromium Cr of 1890 C and nickel Ni of 1453 C compared to 1400-1450 C for stainless steel of type 304.
 Source: britannica.com
Source: britannica.com
Boiling point of Fluorine is -1881C. The chemical elements of the periodic chart sorted by. Melting and boiling points of 3d metals. Fluorines special status also stems from the fluorine factor the ability of this little atom to fine-tune the chemical properties of an entire molecule. Fluorine is found in nature only in the form of its chemical compounds except for trace amounts of the free element in fluorspar that has been subjected to radiation from radiumNot a rare element it makes up about 0065 percent of Earths crust.
 Source: elevise.co.uk
Source: elevise.co.uk
Energy of third ionisation. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Thus higher the stronger the bond between the atoms higher will be the melting point. Helium does not solidify at standard pressure. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active.
 Source: rsc.org
Source: rsc.org
C 4 H 8-1853. Fluorine can form ionic bonds with some elements such as carbon and boron and neon does not tend to form any bonds at all. The melting point is also referred to as liquefaction point solidus or liquidus. Atomic number - Name alphabetically-272. C 3 H 6-1274.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
Molecular size is important but shape is also. Melting point of Fluorine is -2198C. Energy of second ionisation. C 4 H 8-1853. Actual boiling point is 350C 1.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of flourine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.