Melting point of cyclohexane
Home » datasheet » Melting point of cyclohexaneMelting point of cyclohexane
Melting Point Of Cyclohexane. 7C 45F Vapor Density Air1. Carbon dioxide and carbon monoxide may form when heated to decomposition. Molecular size is important but shape is also. The freezing point of the solution is 432 C.
 The Melting Points From Benzene To Cyclohexane A Prime Example Of Dispersion Forces In Action Henry Rzepa S Blog From ch.imperial.ac.uk
The Melting Points From Benzene To Cyclohexane A Prime Example Of Dispersion Forces In Action Henry Rzepa S Blog From ch.imperial.ac.uk
Alkanes comprising of more than three carbon atoms are capable of. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. The presence of a soluble impurity almost always causes a decrease in the melting point expected for the pure compound and a broadening of the. Calculate the molar mass of the unknown solute. Calibration Compound Literature Melting Point C Actual Melting Point C 3 Phenylpropionic Acid 486 43-45 Acetamide 823 69-71 Benzamide 133 103-117 Ice 0 3 Table 6. But actually it is less strained and more stable than.
3 Chemical and Physical Properties Expand this section.
Narcotic effectsPrecautionary Statement Codes. It is a metabolite of cyclohexene oxide and other such compounds. Alkanes comprising of more than three carbon atoms are capable of. In the following diagram cyclohexane represents a low-energy reference point. The deviation of bond angle in cyclohexane molecules is more than in cyclopentane it should be more strained and less reactive than cyclopentane. Two other low-temperature metastable phases III.
 Source: ch.imperial.ac.uk
Source: ch.imperial.ac.uk
Melting point - the temperature at which a solid turns into a liquid. 42880 K Solubility in water. 7C 45F Vapor Density Air1. Calculate the molar mass of the unknown solute. Compound Melting Point C.
Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. Calibration Compound Literature Melting Point C Actual Melting Point C 3 Phenylpropionic Acid 486 43-45 Acetamide 823 69-71 Benzamide 133 103-117 Ice 0 3 Table 6. 95 20C 68F Evaporation Rate BuAc1. 2 Names and Identifiers Expand this section. But actually it is less strained and more stable than.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
86 g100 mL 20 C. The melting point depends on the pressure. Its not a straightforward topic. This process co-forms cyclohexanol and this mixture called KA Oil for ketone-alcohol oil is the main feedstock. The melting and freezing point changes with pressure but normally they are given at 1 atm.
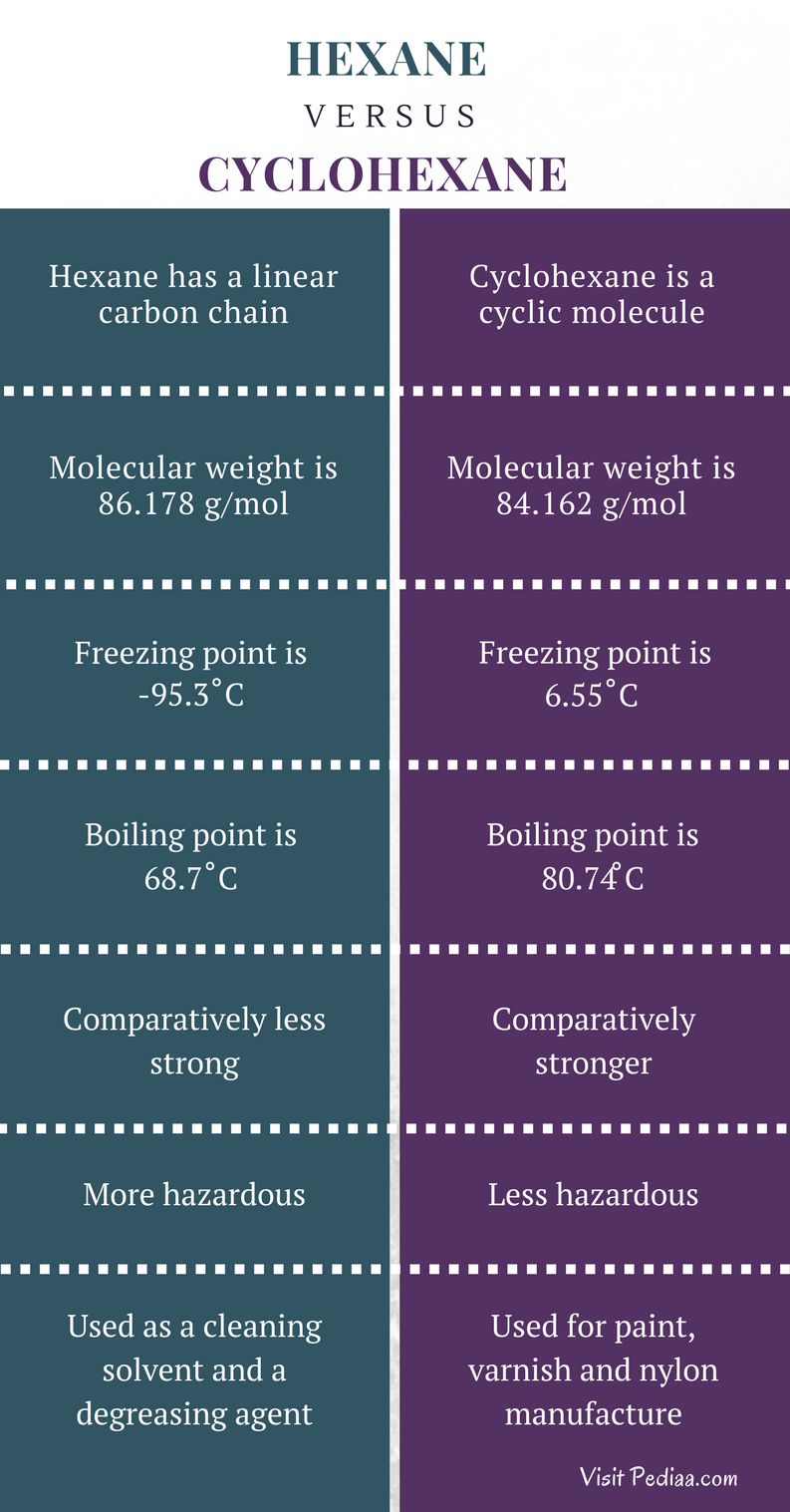 Source: pediaa.com
Source: pediaa.com
The higher the freezing point constant the higher the freezing depression point hence the higher the accuracy. The higher the freezing point constant the higher the freezing depression point hence the higher the accuracy. C 6 H 12 O 2 CH 2 5 CO H 2 O. Cyclohexanone is produced by the oxidation of cyclohexane in air typically using cobalt catalysts. Tf Kf m molality Tf 218 C 0109 mole solutekg Kf.

C 6 H 12 O 2 CH 2 5 CO H 2 O. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Kf for cyclohexane is 200 Ckgmole. It is a metabolite of cyclohexene oxide and other such compounds. The melting point is specific for a given substance.

The freezing point of cyclohexane is 650 C. Addition of hydrogen to cyclohexene produces cyclohexane and releases heat amounting to 286 kcal per mole. For mixtures of compounds as. 101021ja00980a006 This paper covers a classic experiment and is commonly mentioned in undergraduate and graduate organic chemistry or. Compound Melting Point C.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
May be fatal if swallowed and enters airways Danger Aspiration hazardH336 1455. Its prevalence undoubtedly a consequence of its stability makes it the most important of the cycloalkanes. Elle est exprimée. The point of this post is to describe. Melting Point of Recrystallized Benzoic Acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The freezing point of cyclohexane is 650 C. Cyclohexanone is produced by the oxidation of cyclohexane in air typically using cobalt catalysts. 7C 45F Vapor Density Air1. 101021ja00980a006 This paper covers a classic experiment and is commonly mentioned in undergraduate and graduate organic chemistry or. Stable at room temperature in sealed containers.

La chaleur nécessaire pour faire fondre le corps est appelé enthalpie de fusion ou chaleur latente de fusion L f. The melting point is specific for a given substance. The low melting and boiling points of covalent compounds can be explained as below. The deviation of bond angle in cyclohexane molecules is more than in cyclopentane it should be more strained and less reactive than cyclopentane. 86 g100 mL 20 C.
 Source: ddbst.com
Source: ddbst.com
It is a metabolite of cyclohexene oxide and other such compounds. May cause drowsiness or dizziness Warning Specific target organ toxicity single exposure. Cyclohexane is the most widely occurring ring in compounds of natural origin. The low-temperature below 186 K phase II is ordered. The freezing point of cyclohexane is 650 C.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of cyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.