Melting point of cobalt
Home » datasheet » Melting point of cobaltMelting point of cobalt
Melting Point Of Cobalt. The melting point of a substance depends on pressure and is usually specified at standard pressure. 886 grams per cubic centimeter. 0063 nm 3 Isotopes. The melting point of type 7740 Pyrex is 1510F or 820C.
 Wcco Cobalt Tungsten Carbosystem From carbosystem.com
Wcco Cobalt Tungsten Carbosystem From carbosystem.com
Energy of second ionisation. For example the melting point of ice frozen water is 0 C. Value given for diamond form. Metals and Alloys - Melting Temperatures Melting temperatures of common metals and alloys. Electrical Resistivity -6 10x Ω-m. Adding a heat will convert the solid into a liquid with no temperature change.
Product Melting Point o CBoiling Point o CAgate.
Melting Point and Weights of Various Metals and Alloys. The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. So preheat the glass jugs or cups made of pyrex before exposing it to a sudden temperature increase. Initial research on the melting ties in system Fe-Ni-O-S below 20 GPa indicated that geochemically plausible iron alloys significantly reduced the Fe solidus from 2200 to 1150K. The terms melting point or freezing point are often interchanged depending on whether a substance is being heated or cooled. Weight in Troy OzsCu In.
 Source: carbosystem.com
Source: carbosystem.com
Alloy add-ons also suppress. It is also a temperature at which a solid crystal turns into a liquid. Electronic shell Ar 3d 7 4s 2. 0063 nm 3 Isotopes. 2 - Atomic number-259.
 Source: researchgate.net
Source: researchgate.net
As a refractory metal generally the melting point is higher than 1650 with the highest melting point it has good high-temperature strength. From the German word for goblin or evil spirit kobald and the Greek word for mine cobalos. Which essentially implies breaking a few bonds. Whats in a name. At the melting point the solid and liquid phase exist in equilibrium.
 Source: britannica.com
Source: britannica.com
Hence ice melts at low. Melting point of tool steel A2 steel is around 1420C. The kitchenware made of pyrex will never melt unless heat exceeds this limit. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. Helium does not solidify at standard pressure.
 Source: thoughtco.com
Source: thoughtco.com
Standard potential - 028 V Co 2 Co. Thus higher the stronger the bond between the atoms higher will be the melting point. Solubility in H2O. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications. 184 V Co 3 Co 2 Discovered by.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
Melting temperatures of common metals and alloys. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. Melting Point of Tool Steel A2 Steel. For liquids it is known as the freezing point and for solids it is called the melting point. Thus higher the stronger the bond between the atoms higher will be the melting point.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When considered as the temperature of the reverse change from. Melting points for some. For example the melting point of ice frozen water is 0 C. The melting point of a substance depends on pressure and is usually specified at standard pressure. Hence ice melts at low.
 Source: haikudeck.com
Source: haikudeck.com
When considered as the temperature of the reverse change from. Melting temperatures of common metals and alloys. The melting point is the highest temperature at which crystallization may occur. Value given for yellow phosphorus form. 0063 nm 3 Isotopes.
 Source: researchgate.net
Source: researchgate.net
Value given for alpha form. Helium does not solidify at standard pressure. Fahrenheit f Celsius c Admiralty Brass. 3200 K 2927C or 5301F Density. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications.
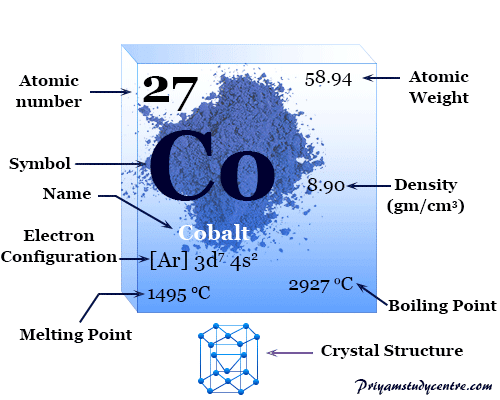 Source: priyamstudycentre.com
Source: priyamstudycentre.com
The chemical elements of the periodic chart sorted by. Phase at Room Temperature. Thus it melts at high temperatures ie 801C whereas ice is a compound comprising of hydrogen bonds whose strength is less than ionic bonds. 184 V Co 3 Co 2 Discovered by. Notes on the Melting Point of particular elements.
Source: theodoregray.com
You can also watch our Melting Points video that explains the melting ranges for various metals. The melting point of type 7740 Pyrex is 1510F or 820C. 0063 nm 3 Isotopes. Adding a heat will convert the solid into a liquid with no temperature change. The melting point of a substance is the temperature at which this phase change occurs.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of cobalt by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.