Melting point of cl
Home » datasheet » Melting point of clMelting point of cl
Melting Point Of Cl. Al 4 C 3. Energy of first ionisation. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. In other words every kilogram of seawater has approximately 35 grams 12 oz of dissolved salts-predominantly sodium Na and chloride Cl- ions are present.
 Melting Point And Boiling Point Elements Group 7 Halogen From elementhalogen.weebly.com
Melting Point And Boiling Point Elements Group 7 Halogen From elementhalogen.weebly.com
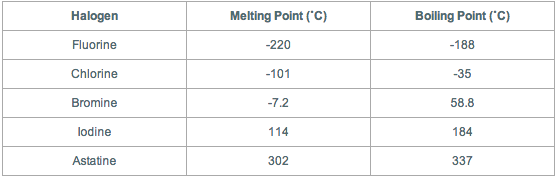
A On the heating curve diagram provided above label each of the following regions. 24553 K-248447 C-415205 F. Microspheres containing the mucoadhesive polymer chitosan hydrochloride with matrix polymer Eudragit RS pipemidic acid as a model drug and agglomeration preventing agent magnesium stearate were prepared by the solvent evaporation method. 86 - Elements in earthcrust-39. Up to date curated data provided by Mathematicas ElementData. Chlorine Cl 2 is a much smaller molecule with comparatively weak van der Waals attractions and so chlorine will have a lower melting and boiling point than sulphur or phosphorus.
Value given for yellow phosphorus form.
The graph shows how melting points and boiling points vary. A sample of water is heated from a liquid at 40 o C to a gas at 110 o C. Melting point C silicon hydride silan SiH 4-185. Notes on the Melting Point of particular elements. Energy of first ionisation. At 20C rotenone is practically insoluble in water 02 mgl see however Loeb and Engstrom-Heg 1970 but readily soluble in chloroform 472 gl and acetone 66 gl and only slightly soluble in ethyl ether 4 gl and ethyl alcohol 2 gl.
 Source: gauthmath.com
Source: gauthmath.com
273 K 0 C Strictly speaking it should be 27315 rather than 273 but the less precise value is acceptable at A Level. The tabular chart on the right is arranged by melting point. Professor Nicholas Golledge in Antarctica says each time his. All icy surfaces in fact contain small puddles of water. Thus higher the stronger the bond between the atoms higher will be the melting point.
 Source: researchgate.net
Source: researchgate.net
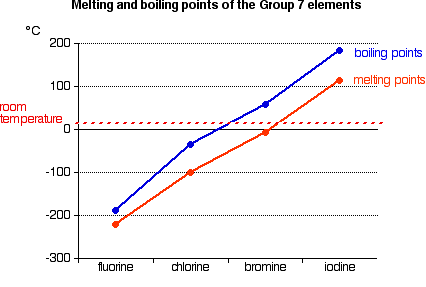
Because salt is soluble in water. The tabular chart on the right is arranged by melting point. 17 - Inventor surname-71. 1 HF l 2 CH 3 Cl l 3 CH 3 F l 4 HCl l ANSWER— HF The higher the boiling Point the stronger the force of attraction between the molecules. Melting point C chlorine.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. Under a pressure of 28 atmospheres. Notes on the Melting Point of particular elements. The temperatures are given in kelvin K. You can easily convert K to C and back again.
 Source: elementhalogen.weebly.com
Source: elementhalogen.weebly.com
Cl 2 O 7 for example can be obtained by dehydrating perchloric acid HClO 4. At 20C rotenone is practically insoluble in water 02 mgl see however Loeb and Engstrom-Heg 1970 but readily soluble in chloroform 472 gl and acetone 66 gl and only slightly soluble in ethyl ether 4 gl and ethyl alcohol 2 gl. En cumplimiento del Reglamento UE 2016679 de Protección de Datos y demás normativa vigente en materia de Protección de Datos se le informa de que sus datos de carácter personal serán tratados por Acciona SA. Melting point C polystyrene C 8 H 8 n. A sample of water is heated from a liquid at 40 o C to a gas at 110 o C.
 Source: chemproject11.weebly.com
Source: chemproject11.weebly.com
For chemistry students and teachers. Melting point - the temperature at which a solid turns into a liquid. Bill Hickman 1613 Nov 19 2021. Antarctic ice sheet may have reached tipping point study reveals. Microspheres containing the mucoadhesive polymer chitosan hydrochloride with matrix polymer Eudragit RS pipemidic acid as a model drug and agglomeration preventing agent magnesium stearate were prepared by the solvent evaporation method.
 Source: chemguide.co.uk
Source: chemguide.co.uk
The chemical element with the lowest melting point is Helium and the element with. 35 - Covalenz radius. Melting point C chlorine. 14025 K-258975 C-434 F. John Margrave a chemistry professor at Rice University explains.
 Source: elevise.co.uk
Source: elevise.co.uk
When going down the each group molecular mass increases which can be reason to have a higher melting and boiling points. All icy surfaces in fact contain small puddles of water. Avenida de Europa 18 Parque empresarial de. 86 - Elements in earthcrust-39. Energy of third ionisation.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
The melting point of rotenote in its orthorhombic form is 165166C. In other words every kilogram of seawater has approximately 35 grams 12 oz of dissolved salts-predominantly sodium Na and chloride Cl- ions are present. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. Up to date curated data provided by Mathematicas ElementData. No Carbonyl present_ Carbonyl present No Aromatic present_ Aromatic present No OH or NH present OH or NH present.

The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Microspheres containing the mucoadhesive polymer chitosan hydrochloride with matrix polymer Eudragit RS pipemidic acid as a model drug and agglomeration preventing agent magnesium stearate were prepared by the solvent evaporation method. The temperatures are given in kelvin K. 86 - Elements in earthcrust-39.
 Source: haikudeck.com
Source: haikudeck.com
Chlorine Cl 2 is a much smaller molecule with comparatively weak van der Waals attractions and so chlorine will have a lower melting and boiling point than sulphur or phosphorus. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. 24553 K-248447 C-415205 F. Melting point C chlorine. Melting Point -5C_____ Boiling Point -5C Index of Refraction nD20 -0005_ Molecular Weight -2amu Formula.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of cl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.