Melting point of chlorine
Home » datasheet » Melting point of chlorineMelting point of chlorine
Melting Point Of Chlorine. At normal atmospheric pressure carbon does not melt when heated it sublimes. Melting point and freezing points thus occur at the same temperature because the change of state involves the same two states liquid-solid. Sample analyzed for equivalent. 1424 at 59F.
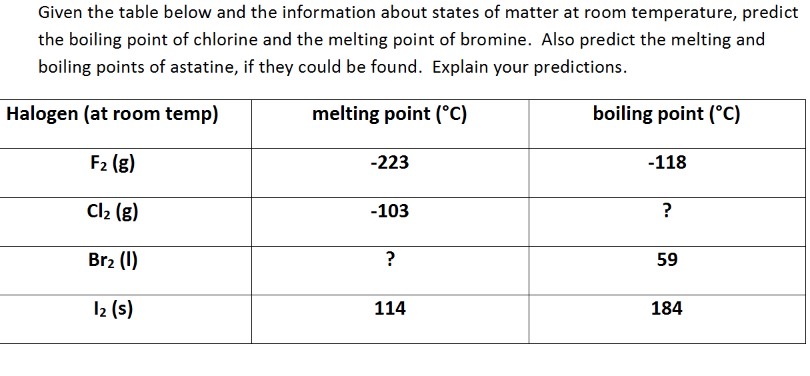 Solved Given The Table Below And The Information About Chegg Com From chegg.com
Solved Given The Table Below And The Information About Chegg Com From chegg.com
Electronic shell Ne 3s 2 3p 5. For example if A is cinnamic acid mp. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. As the molality changes it affects the boiling point and freezing point also known as the melting point of the solution. Comparison of ionic and covalent materials.
Alloy add-ons also suppress the melting range lower.
The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. Use a table to look up. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Thus higher the stronger the bond between the atoms higher will be the melting point. Boiling point of Chlorine is -346C. 17 - Inventor surname-71.
 Source: haikudeck.com
Source: haikudeck.com
The structure of the sample must be altered for the chemical properties to become apparent. 86 - Elements in earthcrust-39. Note that these points are associated with the standard atmospheric pressure. Energy of first ionisation. As the molality changes it affects the boiling point and freezing point also known as the melting point of the solution.
 Source: gauthmath.com
Source: gauthmath.com
The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules. Value given for alpha form. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Let us look at the elements in the ascending order of their melting points. Below the melting point the solid is the more stable state of the two.
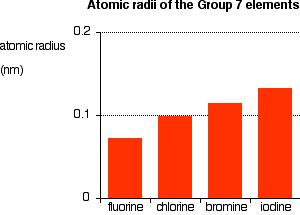 Source: chemguide.co.uk
Source: chemguide.co.uk
If chlorine containing compounds as available chlorine are also expected use a PTFE membranepolypropylene cassette followed by a midget fritted glass bubbler MFGB containing 15 mL 01 Sulfamic Acid for Chlorine Collection. Large amounts of chlorine are used in many industrial processes such as in the production of paper products plastics dyes textiles medicines. 122 ºC the eutectic point is 82 ºC. Melting point The temperature at which the solidliquid phase change occurs. Chlorine is commonly used as an antiseptic and is used to make drinking water safe and to treat swimming pools.
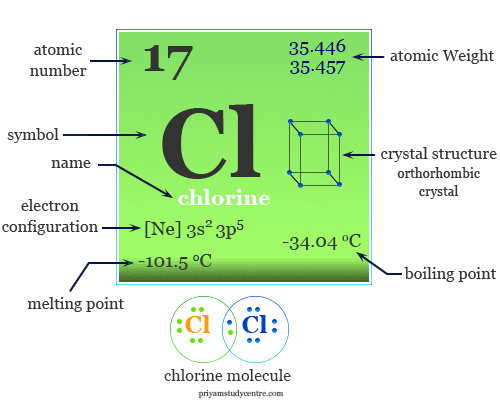 Source: priyamstudycentre.com
Source: priyamstudycentre.com
E ea generally increases across a period row in the periodic table due to the filling of the valence shell of the atom. Notes on the Melting Point of particular elements. Up to date curated data provided by Mathematicas ElementData. 86 - Elements in earthcrust-39. The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules.
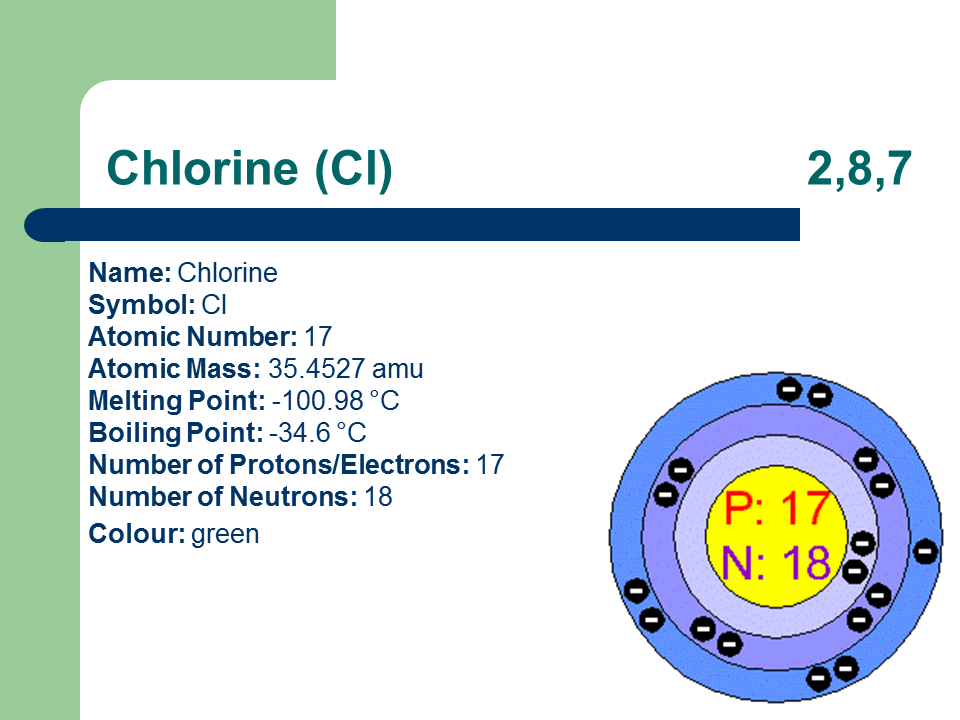
The strength of the van der Waals. Note that these points are associated with the standard atmospheric pressure. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. A higher molality will increase the boiling point and decrease the freezing point of the solution. Boiling point The temperature at which the liquidgas phase change occurs.
 Source: elevise.co.uk
Source: elevise.co.uk
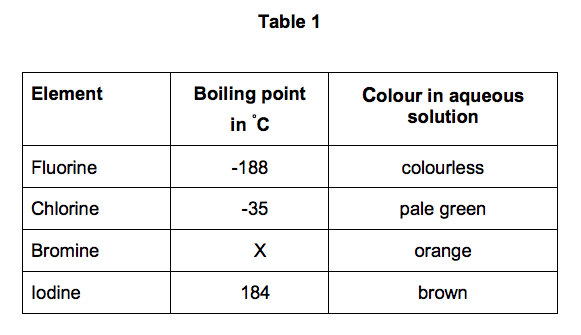
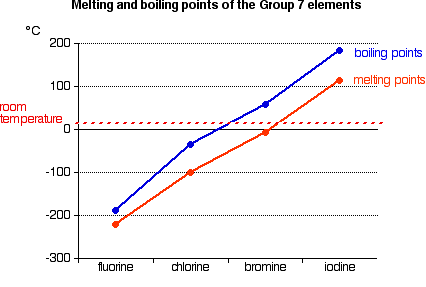
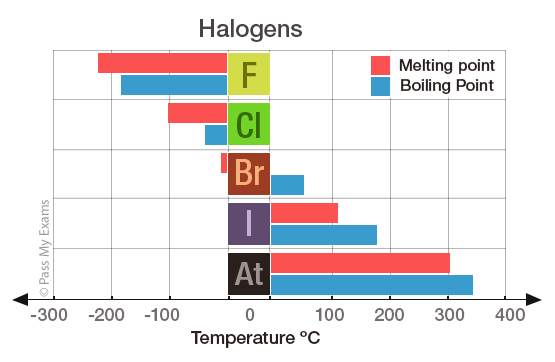
The melting and boiling points of these elements are very low because. You can easily determine what the boiling or freezing point of any solution will be using a simple equation. 35 - Covalenz radius. The chemical element with the lowest melting point is Helium and the. The halogens such as chlorine also exist as diatomic gases by forming covalent bonds.
 Source: chemguide.co.uk
Source: chemguide.co.uk
And the same is true for a sample of B containing a little A. The weight of chlorine which can be dissolved in a given amount of water at a given temperature when the total vapor pressure of chlorine and the water equals a. Chemical properties cannot be determined by touching or viewing a sample. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Iron is a group 6 and period 4 metal.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
The terms melting point or freezing point are often interchanged depending on whether a substance is being heated or cooled. Carl Wilhelm Scheele in 1774. Note the molality m of the solution. At normal atmospheric pressure carbon does not melt when heated it sublimes. Comparison of ionic and covalent materials.
 Source: chegg.com
Source: chegg.com
At normal atmospheric pressure arsenic does not melt when heated. The melting point of a solid and the freezing point of the liquid are normally the same. If chlorine containing compounds as available chlorine are also expected use a PTFE membranepolypropylene cassette followed by a midget fritted glass bubbler MFGB containing 15 mL 01 Sulfamic Acid for Chlorine Collection. Which essentially implies breaking a few bonds. Standard potential - 136 V.

Pure iron Fe has a fixed melting point of 1535 C chromium Cr of 1890 C and nickel Ni of 1453 C compared to 1400-1450 C for stainless steel of type 304. The strength of the van der Waals. If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. Carl Wilhelm Scheele in 1774. Evaporation occurs when water is lost from a substance.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of chlorine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.