Melting point of butane
Home » datasheet » Melting point of butaneMelting point of butane
Melting Point Of Butane. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain the motion of the molecules more severely than in the liquid state. 56 C 1328 F Boiling point of alcohol.
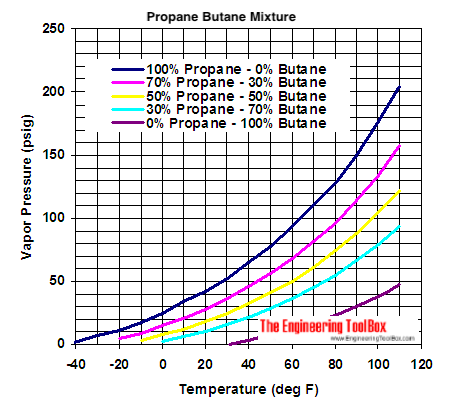 Propane Butane Mixture Evaporation Pressure From engineeringtoolbox.com
Propane Butane Mixture Evaporation Pressure From engineeringtoolbox.com
Hybridization also changes the energy levels of the orbitals. There is certainly a technique for. The molecular sp 3 orbitals are arranged in a tetrahedron with bond angles of 1095 o. At the critical point there is no change of state when pressure is increased or if heat is added. C 8 H 18. C 10 H 22-30.
Butane ˈ b juː t eɪ n or n-butane is an alkane with the formula C 4 H 10Butane is a gas at room temperature and atmospheric pressure.
The presence of a longer. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. It is a gas molecular entity and an alkane. These salient features will generate minimal heat while maintaining a high current flow with minimal voltage drop. Melting point C Density kgm 3 at 20 C Isomers. Highly branched vs.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3. Its the better hands down. N-butane like Puretane butane is highly refined and is the kind of butane we usually think of when we hear the word. Highly branched vs. 5 Related Records Expand this section.
 Source: thermopedia.com
Source: thermopedia.com
Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane CH 4. Butane ˈ b juː t eɪ n or n-butane is an alkane with the formula C 4 H 10Butane is a gas at room temperature and atmospheric pressure. Branched more sphere-like - lower surface area lower boiling point. Intramolecular forces bonding forces exist within molecules and influence the chemical properties. It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point.
 Source: researchgate.net
Source: researchgate.net
Intermolecular forces exist between molecules and influence the physical properties. The triple point of a fluid defines the lowest temperature at which most substances can remain in the liquid state. 1 Structures Expand this section. Below this temperature only solid and gas states are possible in most applications and the boundary between these states is known as the sublimation line. 3732 K Boiling point of ethanol.
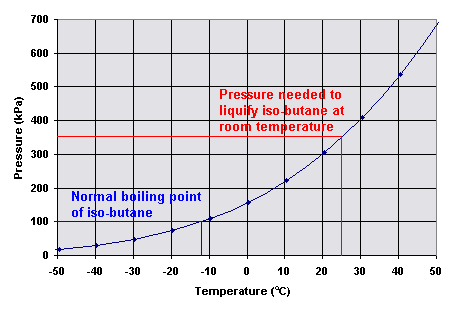 Source: digipac.ca
Source: digipac.ca
The melting or freezing line describes. We can think of H 2 O in its three forms ice water and steam. Hard to describe but if you build a molecule and imagine it on a. The melting point of sodium butanoate is higher than that of butyric acid because the attractive force in sodium butanoate is strong ionic interation. Melting point of some common organic compounds.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
5 Related Records Expand this section. 103 x 45 x 15 inches. Melting point of some common organic compounds. Therefore its melting point is greater than that of butane. Simple geometry puts the angles from the x-axis as 35º below X for CH2Cl2 and only 15º below the X-axis for CHCl3.
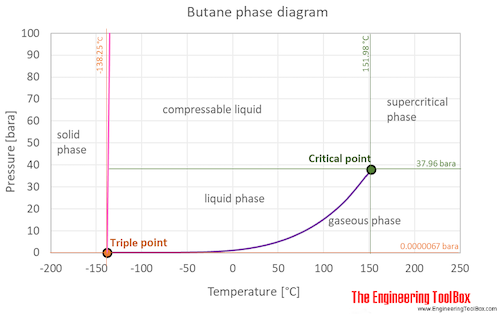 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The curve between the critical point and the triple point shows the butane boiling point with changes in pressure. C 2 H 6. 42 188 201 gas 1 Butane. It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point. The presence of a longer.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
C 12 H 26-10. Thus the melting points of triglycerides reflect their composition as shown by the following examples. C 12 H 26-10. 3 Chemical and Physical Properties Expand this section. The triple point of a fluid defines the lowest temperature at which most substances can remain in the liquid state.
 Source: thermopedia.com
Source: thermopedia.com
If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3. C 6 H 14. It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point. It also shows the saturation pressure with changes in temperature. Its not a straightforward topic.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
The presence of a longer. 103 x 45 x 15 inches. Butane ˈ b juː t eɪ n or n-butane is an alkane with the formula C 4 H 10Butane is a gas at room temperature and atmospheric pressure. C 4 H 10-138-05. The name butane comes from the roots but-from butyric acid named after the Greek word for butter and -aneIt was discovered by the chemist.
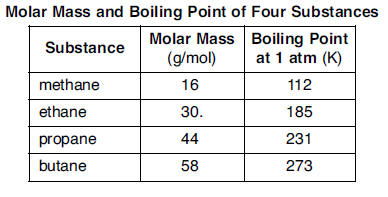 Source: kentchemistry.com
Source: kentchemistry.com
It has a role as a food propellant and a refrigerant. C 3 H 8-190-42. It also shows the saturation pressure with changes in temperature. The melting or freezing line describes. 5 Related Records Expand this section.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of butane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.