Melting point of bromine
Home » datasheet » Melting point of bromineMelting point of bromine
Melting Point Of Bromine. Bromine Br2 CID 24408 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 588C 1378F 332 K. Anthoine Balard in 1826. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
 G A C H P1 S1 Q1 A Elevise From elevise.co.uk
G A C H P1 S1 Q1 A Elevise From elevise.co.uk
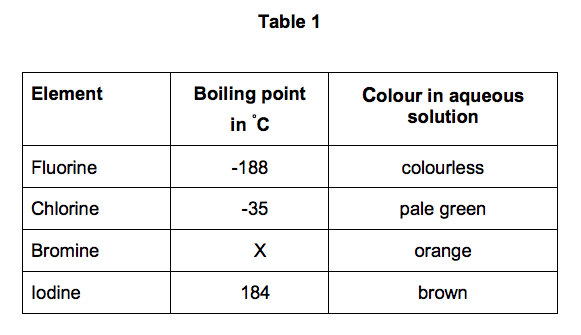
Note that these points are associated with the standard atmospheric pressure. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Natural salt deposits and brines are the main sources of bromine and its compounds. Substance Melting Point. 72 C 450 F - lit. At normal atmospheric pressure carbon does not melt when heated it sublimes.
Value given for hexagonal gray form.
Boiling point The temperature at which the liquidgas phase change occurs. Bromine Br2 CID 24408 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. This is because francium is below caesium in the periodic table so it should have a lower melting point and the difference in melting point. Halogen and noble gases are located in p block of the periodic table. At ambient temperature bromine is a brownish-red liquid. 72C 19F 266 K Period 4 Boiling point.
 Source: youtube.com
Source: youtube.com
Note that these points are associated with the standard atmospheric pressure. 14025 K-258975 C-434 F. The melting point of francium will be around 23C. Bromine Br2 CID 24408 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 72 C 450 F - lit.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The unity used for the melting. Alloy add-ons also suppress the melting range lower. Value given for yellow phosphorus form. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. He found that the salt residues left by evaporating brine from Montpellier France gave an oily red liquid when treated with acid.
 Source: materials.gelsonluz.com
Source: materials.gelsonluz.com
In general boiling is a phase change of a substance from the liquid to the gas phase. Adding a heat will convert the solid into a liquid with no temperature change. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions. The melting point of francium will be around 23C. The melting point depends on the pressure.
 Source: chemguide.co.uk
Source: chemguide.co.uk
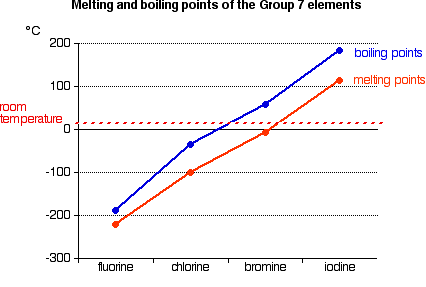
Evaporation occurs when water is lost from a substance. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Even though this is a condensation process we can still use the numerical value of ΔH vap as long as we realize that we must. Bromine Br2 CID 24408 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. What is the energy change when 667 g of Br 2 g condense to a liquid at 595C.
 Source: britannica.com
Source: britannica.com
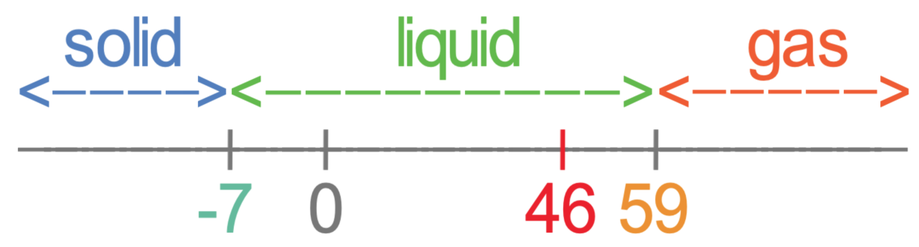
The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Alloy add-ons also suppress the melting range lower. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Notes on the Melting Point of particular elements. Bromine Melting Point and Boiling Point.
 Source: elevise.co.uk
Source: elevise.co.uk
The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. Value given for hexagonal gray form. Adding a heat will convert the solid into a liquid with no temperature change. Notes on the Melting Point of particular elements. Value given for alpha form.

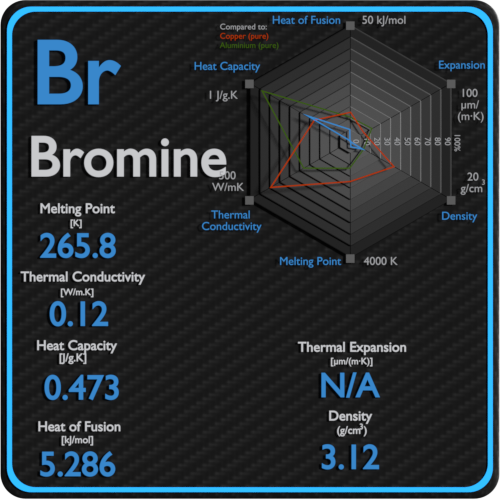
At the melting point the solid and liquid phases exist in equilibrium. Melting point of Bromine is -73C. Now we are going to look melting and boiling points of p block elements from group 13 to group 18. Bromine is at liquid state too. Melting point - 72 C.
 Source: material-properties.org
Source: material-properties.org
Let us look at the elements in the ascending order of their melting points. Halogen and noble gases are located in p block of the periodic table. It is also a temperature at which a solid crystal turns into a liquid. Natural salt deposits and brines are the main sources of bromine and its compounds. Which essentially implies breaking a few bonds.
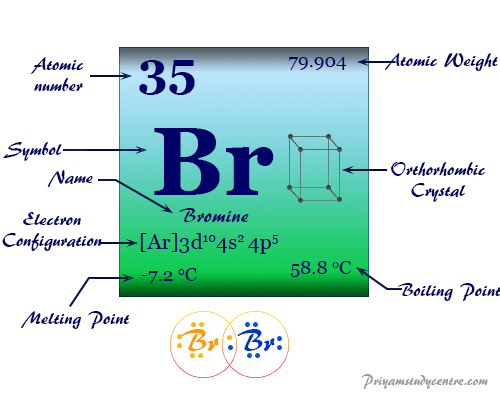 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Symbols Melting Point Name 095 K-27205 C-458 F. Bromine is at liquid state too. When wet clothes are hung out on a clothesline on a sunny day and after a few hours the. Pure iron Fe has a fixed melting point of 1535 C chromium Cr of 1890 C and nickel Ni of 1453 C compared to 1400-1450 C for stainless steel of type 304. Halogen and inert gases melting and boiling points.
 Source: elevise.co.uk
Source: elevise.co.uk
72 C 450 F - lit. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. Substance Melting Point. The boiling point of a substance is the temperature at which this phase change boiling or.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of bromine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.