Melting point of aspirin
Home » datasheet » Melting point of aspirinMelting point of aspirin
Melting Point Of Aspirin. Your sample will be run overnight and data will be. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid. 1385 C Jean-Claude Bradley Open Melting Point Dataset 17267. Place this in an envelope and print your name on it.
 Testing The Purity Of Asprin Youtube From youtube.com
Testing The Purity Of Asprin Youtube From youtube.com
What is the melting point of pure aspirin. In this lab for recrystallization the solution was allowed to cool to room temperature very slowly but was then plunged rapidly. What color does iron III chloride turn a solution of aspirin if it contains traces of salicylic acid. Uses of AspirinAcetylsalicylic acid-C 9 H 8 O 4 Acetylsalicylic acid acts as an inhibitor of cyclo oxygenase. There are two separate reports one for each synthesis and with the acetaminophen. Compare the melting points of your aspirin and pure aspirin.
Determination of the melting point can also give an indication of the purity of a compound as the presence of impurities lowers the melting point and broadens its melting temperature range.
Pure aspirin has a melt point of 135 o C. Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin. Apply the previously learned technique of melting point determination. 15g Melting Point of Impure Aspirin. Add a few crystals of salicylic acid to one test tube. Compare the values you obtain with the literature melting point of pure aspirin.
 Source: youtube.com
Source: youtube.com
Record your melting range of each sample. Uses of AspirinAcetylsalicylic acid-C 9 H 8 O 4 Acetylsalicylic acid acts as an inhibitor of cyclo oxygenase. Add a few crystals of salicylic acid to one test tube. Thus the melting points of triglycerides reflect their composition as shown by the following examples. For detailed mechanism you may visit Mechanism of Acetylsalicylic acid.
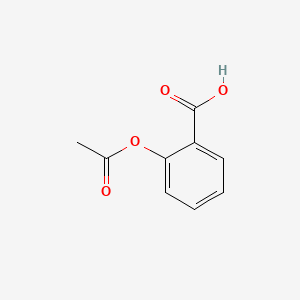
136-138 0118 Mass of Recrystallized Aspirin. Physical Properties of Aspirin It Shows Following Physical Properties Its molar mass is 18016 gmol. You should start with the synthesis of acetaminophen aiming to complete it in the first week. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C. This means that the melting point of aspirin will be relatively high.
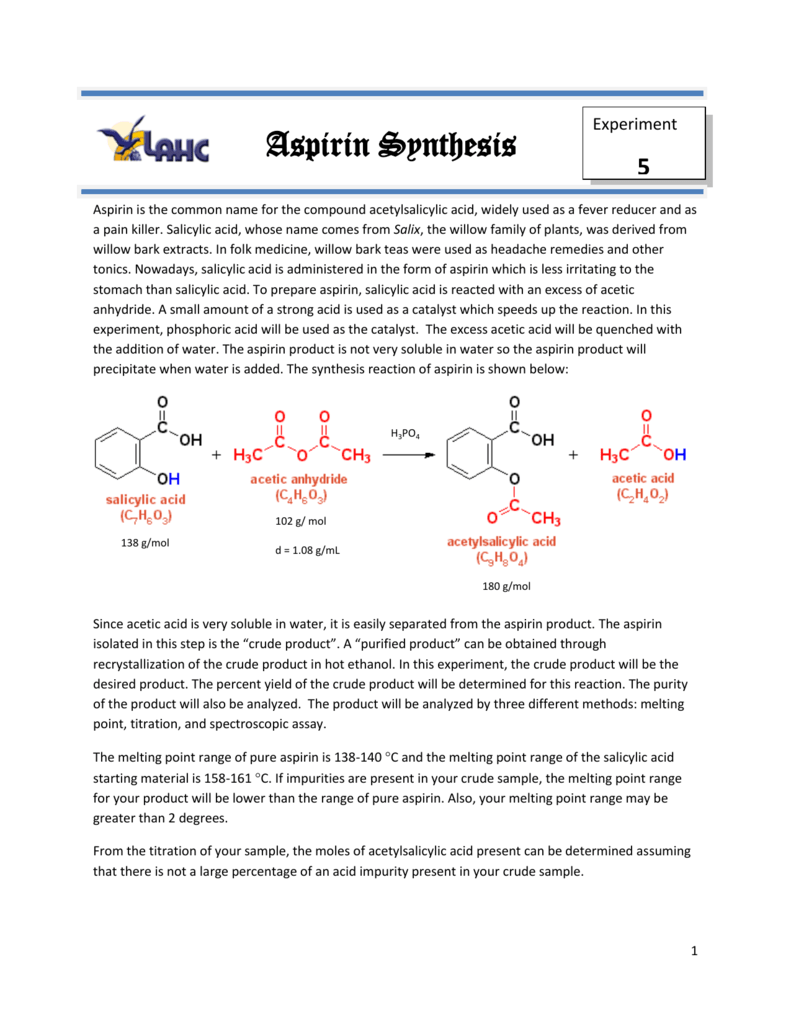 Source: studylib.net
Source: studylib.net
Add a few crystals. Determination of the melting point can also give an indication of the purity of a compound as the presence of impurities lowers the melting point and broadens its melting temperature range. Comment on the purity of your aspirin based on its melting point. Acetylsalicylic acid binds to and acetylates serine residues in cyclooxygenases resulting in decreased synthesis of prostaglandin platelet aggregation and inflammation. Compare the melting points of your aspirin and pure aspirin.
 Source: youtube.com
Source: youtube.com
To confirm that the final product of the synthesis was aspirin an IR spectrum of the product was taken. 15g Melting Point of Impure Aspirin. Determination of the melting point can also give an indication of the purity of a compound as the presence of impurities lowers the melting point and broadens its melting temperature range. It is a white crystalline solid. This agent exhibits analgesic antipyretic and anticoagulant properties.

Its acid dissociation constant pK a is 35 at 25 C 77 F. Aspirin is an orally administered non-steroidal antiinflammatory agent. For the FeCl 3 test the samples do not have to be dry. Aspirin an acetyl derivative of salicylic acid is a white crystalline weakly acidic substance with a melting point of 136 C 277 F and a boiling point of 140 C 284 F. 1600g of aspirin was obtained after the second synthesis.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Permanent dipole permanent dipole bonds may also be formed from the CO bonds on the aspirin molecule. The results displayed a percent yield of 343 from a theoretical yield of about 197g of. Place this in an envelope and print your name on it. This means that the melting point of aspirin will be relatively high. Also your melting point range may be greater than 2 degrees.
 Source: vernier.com
Source: vernier.com
136 C LKT Labs A0819. A large melting point range would suggest an impure sample and ineffective recrystallization process. Place a few. Range of the melting point indicate the purity of the compound. Its melting point is 136.
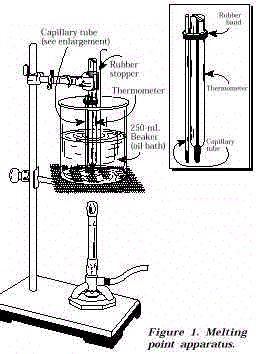 Source: intro.chem.okstate.edu
Source: intro.chem.okstate.edu
Determination of the melting point can also give an indication of the purity of a compound as the presence of impurities lowers the melting point and broadens its melting temperature range. Percent Recovery of Purified Aspirin show calculation below. A small melting point range close to the standard of acetylsalicylic acid would suggest a high purity sample meaning the recrystallization process was effective. 133-136 Melting Point of Recrystallized Aspirin. How do impurities affect melting point of aspirin.
 Source: studylib.net
Source: studylib.net
A large melting point range would suggest an impure sample and ineffective recrystallization process. Then determine the melting point of aspirin using necessary apparatus. If the melting point of the sample is unknown or unavailable a fast run with the. Recrystallization Mass of Impure Aspirin. This means that the melting point of aspirin will be relatively high.
 Source: packerintersections.com
Source: packerintersections.com
Determination of Purity Phenols form a colored complex with the ferric ion. The melting point of pure aspirin is 135C and the melting point of salicylic acid is 158C. Aspirin Purity Testing 5. If impurities are present in your crude sample the melting point range for your product will be lower than the range of pure aspirin. An esterification reaction is the general name for a chemical reaction in which two reactants typically an alcohol and an acid form an ester as the.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point of aspirin by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.