Melting point of antimony
Home » datasheet » Melting point of antimonyMelting point of antimony
Melting Point Of Antimony. Bismuth is thus a useful component of type-metal alloys which make neat clean castings. When considered as the temperature of the reverse change from. The melting point is also referred to as liquefaction point solidus or liquidus. Antimony trichloride is the chemical compound with the formula SbCl 3.
 Yields Melting Points And Elemental Analysis Of Antimony Iii Halide Download Table From researchgate.net
Yields Melting Points And Elemental Analysis Of Antimony Iii Halide Download Table From researchgate.net
Value given for alpha form. At the melting point the solid and liquid phase exist in equilibrium. Melting Point and Weights of Various Metals and Alloys. At normal atmospheric pressure arsenic does not melt when heated. At normal atmospheric pressure carbon does not melt when heated it sublimes. The melting point or rarely liquefaction point of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure.
182 gcm 3 Color.
36 - Vanderwaals radius-108. Antimony metal Antimony powder Stibium Silver-white lustrous hard brittle solid. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. 1587C 2889F 1860 K. Melting and boiling points of 3d. 54 - Year of discovery-62.
 Source: materials.gelsonluz.com
Source: materials.gelsonluz.com
2800 C 55315 K 5360 F Number of ProtonsElectrons. Value given for alpha form. 8 - Boiling point-153. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C. CDC twenty four seven.
 Source: material-properties.org
Source: material-properties.org
Caesium is a soft silvery-gold alkali metal with a melting point of 285 C which makes it one of only five elemental metals that are liquid at or near room temperature. Skip directly to site content Skip directly to page options Skip directly to A-Z link Skip directly to A-Z link Skip directly to A-Z link. Boiling point The temperature at which the liquidgas phase change occurs. At normal atmospheric pressure carbon does not melt when heated it sublimes. At the melting point the solid and liquid phase exist in equilibrium.
 Source: researchgate.net
Source: researchgate.net
The melting point is the highest temperature at which crystallization may occur. Bismuth is thus a useful component of type-metal alloys which make neat clean castings. Helium does not solidify at standard pressure. This list contains the 118 elements of chemistry. When considered as the temperature of the reverse change from.
 Source: britannica.com
Source: britannica.com
Antimony Overview Antimony Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s2 4 d10 5 p3 Abbreviated Electron Configuration. This list contains the 118 elements of chemistry. 441 C 31725 K 11138 F Boiling Point. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. Or a dark-gray lustrous powder.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting point is also referred to as liquefaction point solidus or liquidus. The melting point or rarely liquefaction point of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Melting Point C F Admiralty Brass. It is also a temperature at which a solid crystal turns into a liquid. Symbols Melting Point Name 095 K-27205 C-458 F.
 Source: researchgate.net
Source: researchgate.net
At normal atmospheric pressure arsenic does not melt when heated. Boiling point The temperature at which the liquidgas phase change occurs. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Product Melting Point o CBoiling Point o CAgate. Melting and boiling points of 3d metals.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Melting and boiling points of 3d metals. 2 Second Energy Level. Skip directly to site content Skip directly to page options Skip directly to A-Z link Skip directly to A-Z link Skip directly to A-Z link. Caesium is a soft silvery-gold alkali metal with a melting point of 285 C which makes it one of only five elemental metals that are liquid at or near room temperature. In the case of Antimony the abbreviated electron configuration is Kr 4d10 5s2 5p3.
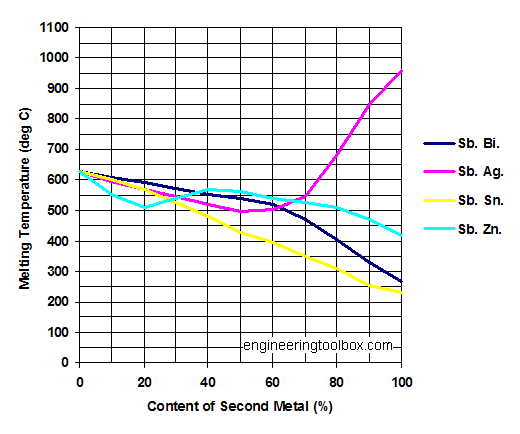 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Monoclinic Density 293 K. At normal atmospheric pressure arsenic does not melt when heated. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. Group 16 elements melting and boiling points. Caesium is a soft silvery-gold alkali metal with a melting point of 285 C which makes it one of only five elemental metals that are liquid at or near room temperature.
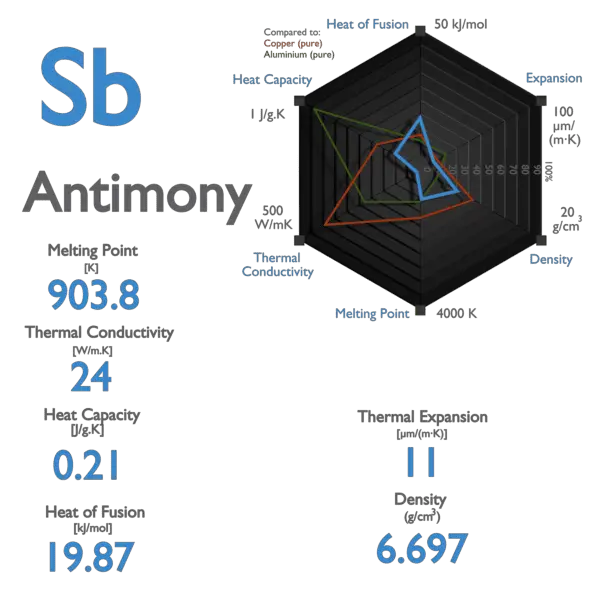 Source:
Source:
Caesium is a soft silvery-gold alkali metal with a melting point of 285 C which makes it one of only five elemental metals that are liquid at or near room temperature. White Atomic Structure. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Melting Point Boiling Point Date of Discovery Crystal Structure. The elements of the periodic table sorted by melting point.
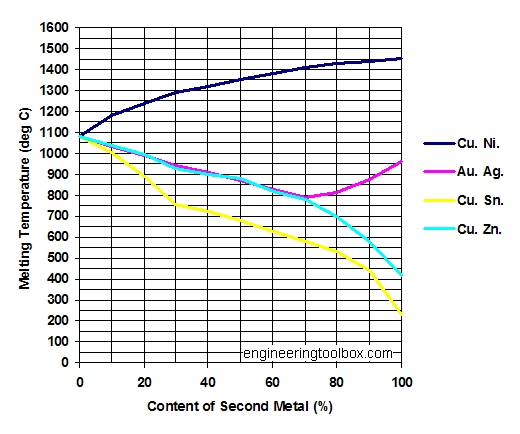 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Antimony trichloride is the chemical compound with the formula SbCl 3. Metallic bismuth is used principally in alloys to many of which it imparts its own special properties of low melting point and expansion on solidification like water and antimony. Tellurium Te has the highest melting point and boiling point. Melting and boiling points of 3d metals. The melting point is specific for a given substanceFor example the melting point of ice frozen water is 0 C.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of antimony by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.