Melting point of 4 chloroaniline
Home » datasheet » Melting point of 4 chloroanilineMelting point of 4 chloroaniline
Melting Point Of 4 Chloroaniline. Less than 1 mgmL at 725 F NTP 1992 National Toxicology Program Institute of. _____ Lab period _____ Results and Calculations to be handed in two days after the next lab period Calculate the percent recovery of 4-chloroaniline from the mixture. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals. A necessity for chemical engg studs.
 4 Chloroaniline 106 47 8 C6h6cln Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
4 Chloroaniline 106 47 8 C6h6cln Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
It has a role as a herbicide a photosystem-II inhibitor a xenobiotic an environmental contaminant and a. D The requirements of this part shall not affect the availability of a waiver under section 121d4 of the Comprehensive Environmental Response Compensation and Liability Act of 1980 CERCLA. Instead it is prepared by reduction of 4-nitrochlorobenzene which in turn is prepared by nitration of. 4-chloroaniline and you can re-take the melting point naphthalene if any is left. 5 Name _____ Date _____ T. E The following hazardous wastes are not subject to any.
E The following hazardous wastes are not subject to any.
It has a role as a herbicide a photosystem-II inhibitor a xenobiotic an environmental contaminant and a. Melting range for 4-chloroaniline _____ Calculate the percent recovery of benzoic acid from the mixture. CRC Handbook of Chemistry and Physics 86TH Edition 2005-2006. Diuron is a member of the class of 3-34-substituted-phenyl-11-dimethylureas that is urea in which both of the hydrogens attached to one nitrogen are substituted by methyl groups and one of the hydrogens attached to the other nitrogen is substituted by a 34-dichlorophenyl group. Index No International Chemical Identification EC No CAS No Classification Labelling Specific Conc. It has a role as a herbicide a photosystem-II inhibitor a xenobiotic an environmental contaminant and a.
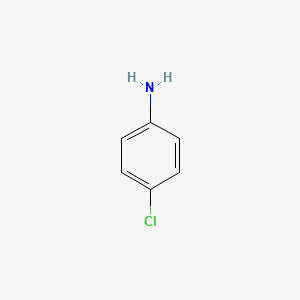
Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. 167 ugmL Burnham Center for Chemical Genomics. Hazardous Substances Data Bank HSDB 326 Solubility. Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis.

In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements. Instead it is prepared by reduction of 4-nitrochlorobenzene which in turn is prepared by nitration of. _____ Lab period _____ Results and Calculations to be handed in two days after the next lab period Calculate the percent recovery of 4-chloroaniline from the mixture. Liquid-Liquid Extraction 4-Chloroaniline and Butyl Butanoate are listed in the appendix of the lab manual - ensure that you are aware of their safetyhandling precautions. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals.
 Source: fishersci.co.uk
Source: fishersci.co.uk
In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements. Academiaedu is a platform for academics to share research papers. Instead it is prepared by reduction of 4-nitrochlorobenzene which in turn is prepared by nitration of. Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. 2-Methylbenzoic acid is likewise toxic by ingestion or inhalation and is also irritating to the skin eyes and respiratory system.
 Source: tcichemicals.com
Source: tcichemicals.com
Liquid-Liquid Extraction 4-Chloroaniline and Butyl Butanoate are listed in the appendix of the lab manual - ensure that you are aware of their safetyhandling precautions. Hazardous Substances Data Bank HSDB 326 Solubility. Academiaedu is a platform for academics to share research papers. 4-Chloroaniline is not prepared from aniline which tends to overchlorinate. 4-chloroaniline and you can re-take the melting point naphthalene if any is left.
 Source: fishersci.fi
Source: fishersci.fi
Less than 1 mgmL at 725 F NTP 1992 National Toxicology Program Institute of. Diuron is a member of the class of 3-34-substituted-phenyl-11-dimethylureas that is urea in which both of the hydrogens attached to one nitrogen are substituted by methyl groups and one of the hydrogens attached to the other nitrogen is substituted by a 34-dichlorophenyl group. Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements. 167 ugmL Burnham Center for Chemical Genomics.
 Source: chemsynthesis.com
Source: chemsynthesis.com
In addition for purposes of 2614a23 and smelting melting and refining furnaces are considered to be solely engaged in metals reclamation if the metal recovery from the hazardous secondary materials meets the same requirements as those specified for metals recovery from hazardous waste found in 266100d1 through 3 of this chapter and if the residuals meet the requirements. 4-Chloroaniline is not prepared from aniline which tends to overchlorinate. Less than 1 mgmL at 725 F NTP 1992 National Toxicology Program Institute of. Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. It has a role as a herbicide a photosystem-II inhibitor a xenobiotic an environmental contaminant and a.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Enter the email address you signed up with and well email you a reset link. It has a role as a herbicide a photosystem-II inhibitor a xenobiotic an environmental contaminant and a. Liquid-Liquid Extraction 4-Chloroaniline and Butyl Butanoate are listed in the appendix of the lab manual - ensure that you are aware of their safetyhandling precautions. Iv The wastes no longer exhibit a prohibited characteristic at the point of land disposal ie placement in a surface impoundment. Diuron is a member of the class of 3-34-substituted-phenyl-11-dimethylureas that is urea in which both of the hydrogens attached to one nitrogen are substituted by methyl groups and one of the hydrogens attached to the other nitrogen is substituted by a 34-dichlorophenyl group.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. CRC Press Taylor Francis Boca Raton FL 2005 p. Instead it is prepared by reduction of 4-nitrochlorobenzene which in turn is prepared by nitration of. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals. D The requirements of this part shall not affect the availability of a waiver under section 121d4 of the Comprehensive Environmental Response Compensation and Liability Act of 1980 CERCLA.
 Source: tcichemicals.com
Source: tcichemicals.com
Hazardous Substances Data Bank HSDB 326 Solubility. Index No International Chemical Identification EC No CAS No Classification Labelling Specific Conc. CRC Handbook of Chemistry and Physics 86TH Edition 2005-2006. Heat of fusion at melting point 236 kJmol. 5 Name _____ Date _____ T.
 Source: trc-canada.com
Source: trc-canada.com
4-chloroaniline and you can re-take the melting point naphthalene if any is left. Melting range for 4-chloroaniline _____ Calculate the percent recovery of benzoic acid from the mixture. 167 ugmL Burnham Center for Chemical Genomics. 2-Methylbenzoic acid is likewise toxic by ingestion or inhalation and is also irritating to the skin eyes and respiratory system. CRC Press Taylor Francis Boca Raton FL 2005 p.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of 4 chloroaniline by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.