Melting point of 1 methylcyclohexene
Home » datasheet » Melting point of 1 methylcyclohexeneMelting point of 1 methylcyclohexene
Melting Point Of 1 Methylcyclohexene. It is when certain compounds have similar. In a relatively flat alkene such as 1-methylcyclohexene addition of the radical will occur with equal probability from either face. BRILLIANT PUBLIC SCHOOL SITAMARHI Affiliated up to 2 level to CBSE New Delhi Class-XI IIT-JEE Advanced Chemistry Study Package Session. 2 Draw the structure of a different cyclic alkene formed from carbocation D.
 Methylenecyclohexane Wikipedia From en.wikipedia.org
Methylenecyclohexane Wikipedia From en.wikipedia.org
Academiaedu is a platform for academics to share research papers. 3 Carbocation D can undergo a type of reaction called a. 4 marks 0 7. It derives from a hydride of a p-menthane. It is a monoterpene and a cycloalkene. The bond could break two different ways after all.
They are unsaturated hydrocarbons.
Limonene is a monoterpene that is cyclohex-1-ene substituted by a methyl group at position 1 and a prop-1-en-2-yl group at position 4 respectively. Their melting and boiling points decrease down the group. The question is which atom of the double bond does the free radical attack. Normal E2 Reactions Follow Zaitsevs Rule Giving The More Substituted Alkene. BRILLIANT PUBLIC SCHOOL SITAMARHI Affiliated up to 2 level to CBSE New Delhi Class-XI IIT-JEE Advanced Chemistry Study Package Session. A Fundamental Equation for Water Covering the Range From the Melting Line to 1273 K at Pressures up to 25000 MPa J.
Source: chemspider.com
CrabtreeThis air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group. It is when certain compounds have similar. The D-isomer occurring more commonly in nature as the fragrance of oranges is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The bond could break two different ways after all.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Crabtrees catalyst is an organoiridium compound with the formula C 8 H 12 IrPC 6 H 11 3 C 5 H 5 NPF 6It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions developed by Robert H. You should expect that the more substituted alkene will be formed if at all possibleLike in the elimination reaction below for instance we get 80 of the tetrasubstituted alkene Zaitsev more substituted because there are 4. 4 marks 0 7. The D-isomer occurring more commonly in nature as the fragrance of oranges is a flavoring agent in food manufacturing. Limonene is a monoterpene that is cyclohex-1-ene substituted by a methyl group at position 1 and a prop-1-en-2-yl group at position 4 respectively.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is a monoterpene and a cycloalkene. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest exam pattern. We have provided Alcohols Phenols and Ethers Class 12 Chemistry MCQs Questions with Answers to help students understand the concept. The D-isomer occurring more commonly in nature as the fragrance of oranges is a flavoring agent in food manufacturing.
 Source: us.vwr.com
Source: us.vwr.com
In a relatively flat alkene such as 1-methylcyclohexene addition of the radical will occur with equal probability from either face. Crabtrees catalyst is an organoiridium compound with the formula C 8 H 12 IrPC 6 H 11 3 C 5 H 5 NPF 6It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions developed by Robert H. Their melting and boiling points decrease down the group. The bond could break two different ways after all. A Fundamental Equation for Water Covering the Range From the Melting Line to 1273 K at Pressures up to 25000 MPa J.
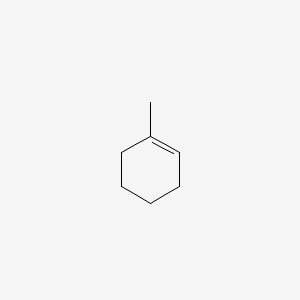
It is a monoterpene and a cycloalkene. It is a monoterpene and a cycloalkene. Data 18 1537-1564 1989. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest exam pattern. The question is which atom of the double bond does the free radical attack.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
CrabtreeThis air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group. 1-methylcyclohexene via carbocation D by drawing the structure of the missing intermediate all necessary curly arrows. Data 18 1537-1564 1989. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest exam pattern. We have provided Alcohols Phenols and Ethers Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It derives from a hydride of a p-menthane. Academiaedu is a platform for academics to share research papers. It is a monoterpene and a cycloalkene. It derives from a hydride of a p-menthane. 13 13 Turn over IBMJun1774042 Do not write outside the box 0 7.
 Source: en.wikipedia.org
Source: en.wikipedia.org
They are linked together exclusively by single bonds. Normal E2 Reactions Follow Zaitsevs Rule Giving The More Substituted Alkene. We have provided Alcohols Phenols and Ethers Class 12 Chemistry MCQs Questions with Answers to help students understand the concept. Their melting and boiling points decrease down the group. 4 marks 0 7.
 Source: markedbyteachers.com
Source: markedbyteachers.com
1-methylcyclohexene via carbocation D by drawing the structure of the missing intermediate all necessary curly arrows. It is when certain compounds have similar. Give the major products of the following reaction. A Fundamental Equation for Water Covering the Range From the Melting Line to 1273 K at Pressures up to 25000 MPa J. We have provided Alcohols Phenols and Ethers Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The D-isomer occurring more commonly in nature as the fragrance of oranges is a flavoring agent in food manufacturing. ρg cm3 09998189 0. 1-methylcyclohexene via carbocation D by drawing the structure of the missing intermediate all necessary curly arrows. Academiaedu is a platform for academics to share research papers. Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene and is the major component in the oil of citrus fruit peels.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point of 1 methylcyclohexene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.