Melting point mgcl2
Home » datasheet » Melting point mgcl2Melting point mgcl2
Melting Point Mgcl2. If individual is experiencing respiratory discomfort or irritation remove to fresh air. Total Valence electron for NaCl lewis. MgCl2 NaCl AlCl3 c. Section V Fire Fighting Measures.
 Magnesium Chloride Ice Melt Ice Melter Distributor Salt Supplier Kissner From kissner.com
Magnesium Chloride Ice Melt Ice Melter Distributor Salt Supplier Kissner From kissner.com
Often a concrete-safe and pet-safe ice melt option. An ion and a water molecule. Metalssonorous Non metalnon sonorous. Melting LightCycler 480 High Resolution Melting Master 04 909 631 001 5 mL 5 x 1 mL 2x concentrated 25 mL MgCl2 stock solution for 500 20 µL reactions Hybridization Probes LightCycler 480 Genotyping Master 04 707 524 001 15 mL 5x concentrated Master Mix for 250 20 µL reactions Hydrolysis Probes TaqMan Probes or Universal Probe Library UPL LightCycler 480 Probes Master. Learn how by reading How to Seal Concrete. All metals except mercury exist as solids at room temperature.
Often a concrete-safe and pet-safe ice melt option.
You can also protect your driveway year-round. Na O2 Na2O. For laboratory and manufacturing use only. Using the same volume of. AlCl3 NaCl MgCl2 d. All questions are of MCQ types here solutions of each question are explained by the.
 Source: scbt.com
Source: scbt.com
Visit BYJUS to understand the properties structure and uses of Magnesium Chloride. Ii K is larger than Na so NaCl has a higher lattice energy and a higher melting point than KCl. Chemical properties of metals and non metals. Answer 1 of 4. How do you tell if a compound is solid liquid gas or aqueous.
 Source: kissner.com
Source: kissner.com
The rest are liquids or low melting point solids. Which of the following solids would have the highest melting point. Metals react with oxygen to form metal oxide. When hit they produce sound. This increase in size means that the oppositely charged ions in KCl are further part thanthose in NaCl and therefore it takes less energy to break up the ionic bond in KCl which we see as a reduction in the melting point.
 Source: researchgate.net
Source: researchgate.net
It has a coordinate geometry of octahedral for Na and Cl-. A cation and a water molecule. A KI B KBr C KCl D KF. Using the same volume of. - 2592 C MgCl2- colorless crystalline soluable in water H2- forms covalent bonds diatomic atom Energy of first ionisation.
 Source: sahay.guru
Source: sahay.guru
Platinum is an allotrope of carbon is the hardest natural substance known and has a very. For non-molecular substances such as table salt we represent the. It has a melting point of 8007 C and a boiling point of 1465 C. Why NaCl has more lattice energy than KCl. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Using the same volume of. If we notice the nature of oxides. Which of the following solids would have the highest melting point. An ion and a polar molecule. If individual is experiencing respiratory discomfort or irritation remove to fresh air.
 Source: chem-solutions-9701.blogspot.com
Source: chem-solutions-9701.blogspot.com
I start my students with learning the standard states of the elements. Iondipole forces always require a. If discomfort or irritation persists get medical attentionadvice. Visit BYJUS to understand the properties structure and uses of Magnesium Chloride. This indicates that the initial bulk MgCl 2 layers are too densely packed to accept a H 2 O molecule and the created H 2 O water layer in the cavity is needed to deform the densely packed layers and create a disordered region of salt and water.
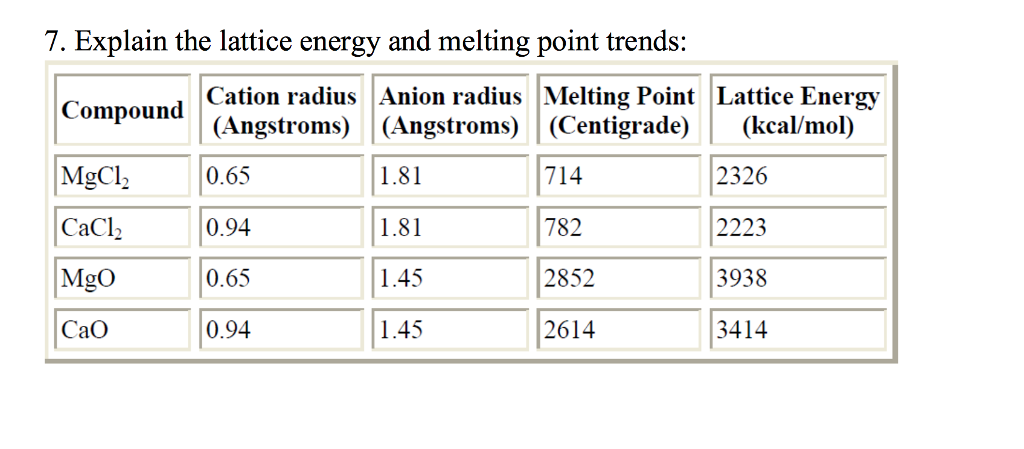 Source: chegg.com
Source: chegg.com
17944 19659 Initial pressure kPa 100. The sodium chloride has a higher melting point because of the greater charges of the ions and hence the greater force of attractions between them. This formula implies that the water molecules consist of 2 hydrogen and 1 oxygen atoms. A KI B KBr C KCl D KF. Metal oxygen metal oxide.
 Source: oneclass.com
Source: oneclass.com
Mg O2 MgO. Which of the following solids would have the lowest melting point. Which of the following solids would have the highest melting point. When hit they produce sound. Over time one becomes familiar with certain substances.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Mg2 Na H-Br N 2 10. When hit they produce sound. AlCl3 NaCl MgCl2 d. A NaI B NaF C MgO D MgCl2 E KF. Melting LightCycler 480 High Resolution Melting Master 04 909 631 001 5 mL 5 x 1 mL 2x concentrated 25 mL MgCl2 stock solution for 500 20 µL reactions Hybridization Probes LightCycler 480 Genotyping Master 04 707 524 001 15 mL 5x concentrated Master Mix for 250 20 µL reactions Hydrolysis Probes TaqMan Probes or Universal Probe Library UPL LightCycler 480 Probes Master.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Order of increasing melting point. The group 2 elements do exhibit some anomalies however. 008 012 Volume of flask mL 157. When hit they produce sound. Order of increasing melting point.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point mgcl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.