Melting point hf
Home » datasheet » Melting point hfMelting point hf
Melting Point Hf. Below the melting point the solid is the more stable state of the two. Value given for monoclinic beta form. Garnet is a possible Hf-source since it is a reservoir of Lu which decays to 176 Hf and could be released into the melt during the melting history Tang et al 2014. The liquid may be cooled by putting the boiling tube in a beaker of cold water or just leaving it in the air.
 Supplemental Topics From www2.chemistry.msu.edu
Supplemental Topics From www2.chemistry.msu.edu
Melting point C. Note that the compositions between 0 melting and where the dark line intersects the En-Di join are SiO 2 oversaturated liquids and those from this point up to 100 melting are SiO 2 undersaturated. Materials with strong bonds between atoms will have a high melting temperature. Atomic number - Name alphabetically-272. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. Hydrogen is the most common element in the universe making up about 75 of its mass.
Answer 1 of 7.
Notes on the Melting Point of particular elements. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. At the melting point the solid and liquid phase exist in equilibrium. Br 2Br2 59 C and IClICl 97 C Author. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Atomic number - Name alphabetically-272.
 Source: chegg.com
Source: chegg.com
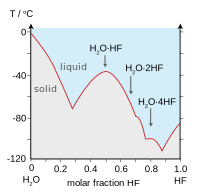
122 ºC the eutectic point is 82 ºC. H 2 SO 4. Based on the geochemistry and Sr-Nd-Hf-O isotope data the quartz monzonite porphyry with elevated δ 18 O 6674 high εNdt 58 and high εHft 5084 formed by the partial melting of juvenile material ultimately derived from depleted mantle which had been modified by melts derived from sediment on the downgoing slab. Salol has a melting point of about 45C and stearic acid has a melting point of about 69C. H 2 TeO 3.
 Source: en.wikipedia.org
Source: en.wikipedia.org
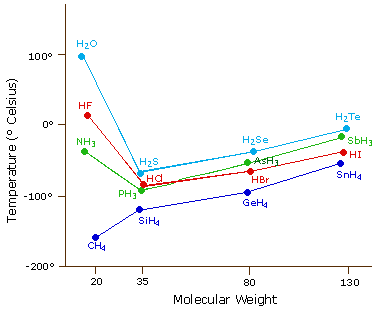
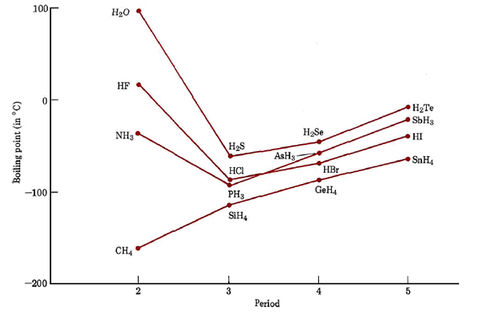
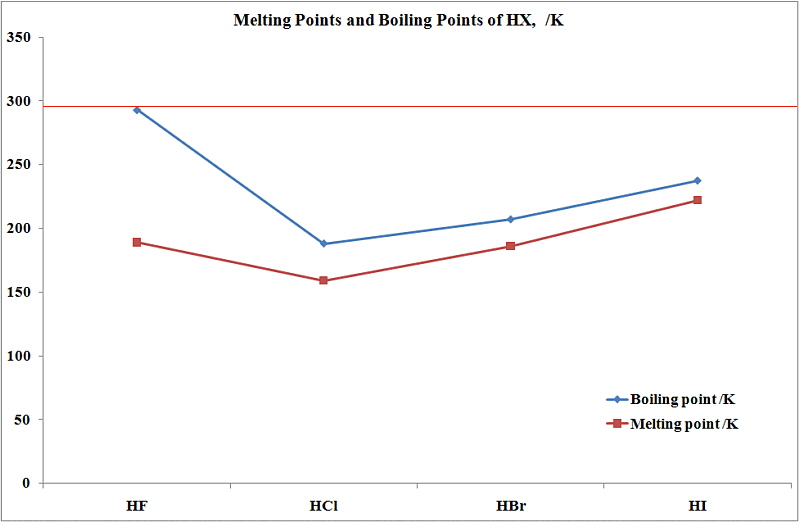
86 - Inventor surname-35. At the melting point the two phases of a substance liquid and vapor have identical free energies and therefore are equally likely to exist. Below is a table of the melting points boiling points and densities of the elements. HFHF 20 C and HClHCl -85 C b. Caesium has physical and chemical properties similar to those of rubidium and potassium.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Below the melting point the solid is the more stable state of the two. The lowest mixture melting point e is called the eutectic point. 2 - Atomic number-259. 8 - Boiling point-153. That was Eric Scerri revealing the powers of Hafnium.
 Source: youtube.com
Source: youtube.com
17 - Elements in earthcrust. Now next week we meet the King of the elements. 24553 K-248447 C-415205 F. H 2 SO 4. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

Materials with strong bonds between atoms will have a high melting temperature. For example if A is cinnamic acid mp. If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. Adding a heat will convert the solid into a liquid with no temperature change. Let us look at the elements in the ascending order of their melting points.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The melting point or rarely liquefaction point of a substance is the temperature at which it changes state from solid to liquid. Let us look at the elements in the ascending order of their melting points. So long as some of the liquid is left behind liquids can be extracted at any time during the melting event and have compositions anywhere along the dark like between 0 melting and 100 melting. H 3 PO 4. A Periodic table to view elements in 3D.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
So long as some of the liquid is left behind liquids can be extracted at any time during the melting event and have compositions anywhere along the dark like between 0 melting and 100 melting. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Hydrogen is the most common element in the universe making up about 75 of its mass. 17 - Elements in earthcrust. Helium-27220 under pressure.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Now next week we meet the King of the elements. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. Under a pressure of 28 atmospheres. Br 2Br2 59 C and IClICl 97 C Author. 8 - Boiling point-153.
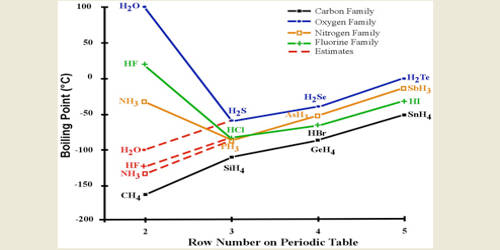 Source: qsstudy.com
Source: qsstudy.com
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. Under a pressure of 28 atmospheres. So long as some of the liquid is left behind liquids can be extracted at any time during the melting event and have compositions anywhere along the dark like between 0 melting and 100 melting. H 2 SO 4.
 Source: en.wikipedia.org
Source: en.wikipedia.org
HFHF 20 C and HClHCl -85 C b. 122 ºC the eutectic point is 82 ºC. 14025 K-258975 C-434 F. If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C. They are easily melted in a boiling tube placed in a beaker of hot water.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title melting point hf by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.