Melting point hexane
Home » datasheet » Melting point hexaneMelting point hexane
Melting Point Hexane. Hexane-16-diol HOCH26OH or C6H14O2 CID 12374 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. This is the reason why it is regarded as a good solvent. Freezing point - the temperature at which a liquid turns into a solid. 180 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air.
 Why Does Neopentane Have A Higher Melting Point Than N Pentane Chemistry Stack Exchange From chemistry.stackexchange.com
Why Does Neopentane Have A Higher Melting Point Than N Pentane Chemistry Stack Exchange From chemistry.stackexchange.com
180 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air. Better stacking higher melting point. In this experiment you will combine both spectroscopy and qualitative tests to identify an unknown organic compound. As the molecular weight increases the melting point increases. N-Hexane is a chemical made from crude oil. The properties of some of these solvents are detailed in the following subsections.
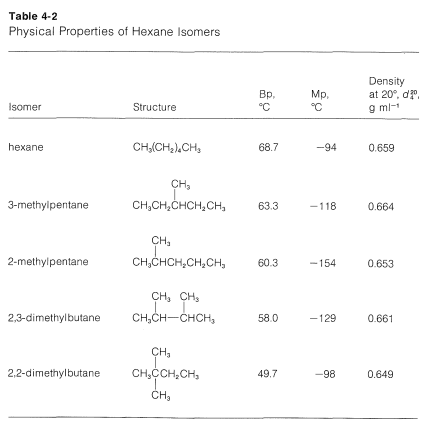
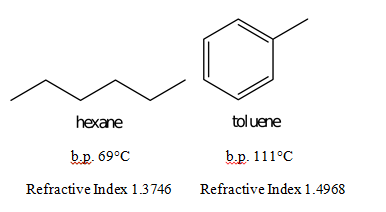
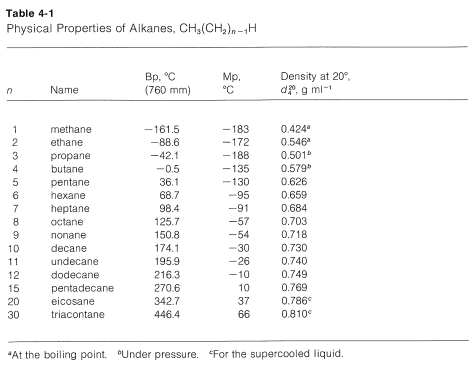
Hexane C 6H 14 mw86 has a boiling point of 68º.
The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role. Stability and Reactivity Stability. The major use for solvents containing n-Hexane is. Also relative molecular mass 1 is very low. It is highly flammable and its vapors can be explosive. At the melting point the solid and liquid phases exist in equilibrium.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Number of carbon MeltingBoiling point Alkane Alkene Alkyne 2 Melting point ethane -183. Boiling point of water. Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice. For this experiment the possible categories of the unknown. Hexane is a significant constituent of gasolineIt is a colorless liquid odorless when pure and with boiling points approximately 69 C 156 F.
 Source: thermopedia.com
Source: thermopedia.com
Mercury Hg has the lowest melting point -3883 0 C because mercury has a very weak metallic lattice. The melting point of the purified derivative allows identification of the unknown. Hexane undergoes combustion reaction readily to form carbon dioxide and water molecules. The melting point is the highest temperature at which crystallization may occur. At the melting point the solid and liquid phases exist in equilibrium.

N-Hexane is a chemical made from crude oil. Furthermore water is often referred to as the universal solvent because it is known to. Most of then-Hexane used in industry is mixed with similar chemicals called solvents. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. 240 oC No melting was observed prior to decomposition Partition Coefficient.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Which element has the lowest melting point element in periodic table. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed. Helium exist as atoms. Also relative molecular mass 1 is very low. Boiling point of water.
 Source: odinity.com
Source: odinity.com
This is a demonstration of colligative properties which can be rationalized by the lowering of the vapor pressure of pure liquids due to the presence of impurities. Look at these three examples. Mark each of the following statements as TRUE or FALSE. Water has the ability to dissolve a large variety of substances. Also relative molecular mass 1 is very low.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The melting point is specific for a given substance. Branching decreases melting point and boiling point. This is a demonstration of colligative properties which can be rationalized by the lowering of the vapor pressure of pure liquids due to the presence of impurities. Boiling and melting point. Its also about surface area.
N-Hexane is a chemical made from crude oil. 2C 6 H 14 19O 2 12CO 2 14H 2 O. Its also about surface area. Water is a polar protic solvent with the chemical formula H 2 O. For this experiment the possible categories of the unknown.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The melting and freezing point changes with pressure but normally they are given at 1 atm. Puren-Hexane is used in laboratories. Stable at room temperature in sealed containers. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed.
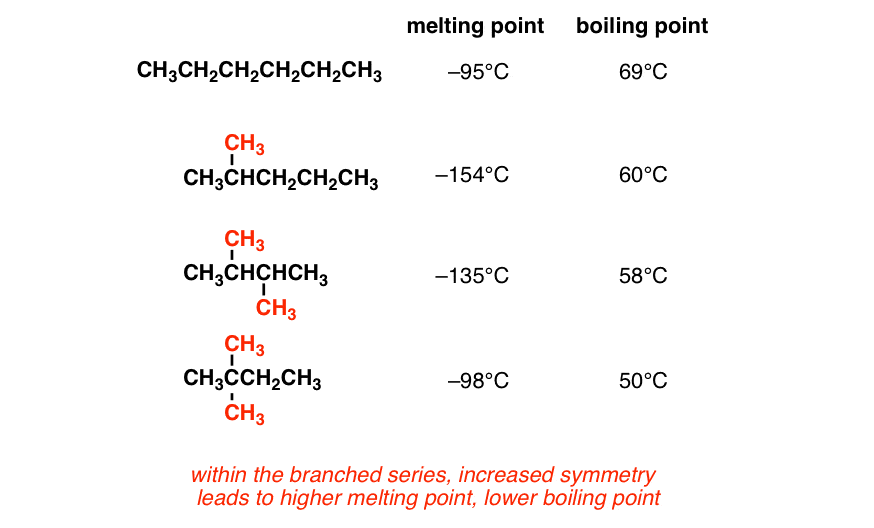 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Freezing point - the temperature at which a liquid turns into a solid. But it gets more complicated. The melting point is also referred to as liquefaction point solidus or liquidus. This is the reason why it is regarded as a good solvent. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg.
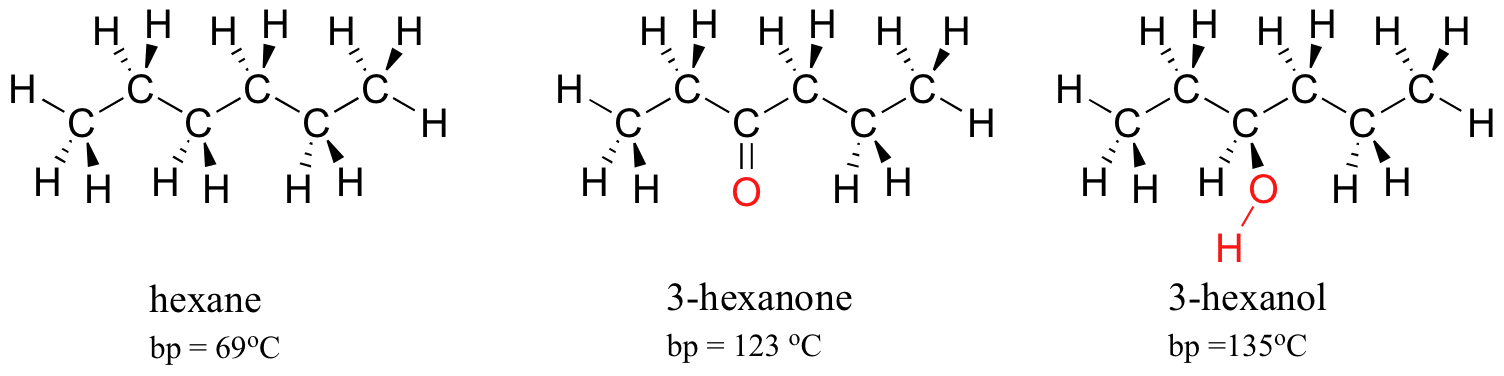 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
Better stacking higher melting point. N-Hexane is a chemical made from crude oil. Hexane-16-diol HOCH26OH or C6H14O2 CID 12374 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. The major use for solvents containing n-Hexane is. Hexane is a significant constituent of gasolineIt is a colorless liquid odorless when pure and with boiling points approximately 69 C 156 F.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title melting point hexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.